Channels lising page
All videos archived of ChemicalForce

WMaOHhmZawk | 08 Jan 2026
Hi! In this video, we’re going to take a look at a few more reactions between anhydrous perchloric acid and junk food. And of course, you’ll also see my cat at the beginning and at the end :D 0:00 INTRO 0:27 Chicken skin test 0:46 Chips test 2:08 Bread crackers 2:45 Love is... 3:34 Big can of Pringles 4:02 Comparison with very cold acid 5:07 Olive oil 5:38 Cheetos test 6:13 Cheetos and potato 6:51 Cheetos and apple 7:29 Cheetos and pomegranate 8:20 Cheetos and pumpkin 9:54 Strong tea 11:09 OUTRO https://www.patreon.com/ChemicalForce

fapWo77XZEU | 05 Dec 2025
Hi! Before publishing the videos about perchloric acid HClO4 and xenon difluoride XeF2, I’m releasing one where both of these super reactive reagents are involved. You can also see a few photos from my upcoming book featuring chemical reaction photography :D 0:00 Intro 0:16 Decomposition of boric acid in a muffle furnace 0:31 Preparation of a mixture of B2O3 and Na 1:47 Boron extraction 2:31 Boron reactivity test with nitric acid 3:15 Reaction of boron and potassium permanganate 3:52 Reaction of boron and lead dioxide 4:27 Reaction of boron and selenic acid 5:02 Anhydrous perchloric acid reactions teaser 5:11 Reaction of boron and Anhydrous perchloric acid HClO4 100% 5:42 Reaction of boron and xenon difluoride in the test tube 6:33 Reaction between xenon diflouride and decaborane 7:13 Decaborane and fuming perchloric acid 7:59 I love my patrons ❤️ https://www.patreon.com/ChemicalForce

kFCp4pE_iZs | 08 Oct 2025
In this video, I make siloxene, a crazy glowing chemical indicator with unique properties, and then mix it with xenon difluoride. __________ 0:00 Demonstration of magnesium and calcium silicides 0:47 Intro 0:57 Synthesis of siloxene 1:46 Combustion of siloxene 2:24 Spontaneous ignition of siloxene in Ozone 4:06 Spontaneous ignition of siloxene in Chlorine 4:24 Siloxene and the acid test 4:50 Siloxene and fuming nitric acid 5:31 Siloxene and selenic acid 5:55 Xenon difluoride demonstration XeF2 6:06 Xenon difluoride and Siloxene reaction 6:59 Siloxane as a chemiluminescent indicator in analytical chemistry __________ ❤️ ❤️ ❤️ Your support inspire me to continue producing exotic chemical content! Patreon: https://www.patreon.com/ChemicalForce ❤️ ❤️ ❤️ __________

FlAAlPQhD2I | 18 Aug 2025
In this video I'll show you a little about magnesium nitride Mg3N2, and we will also see what happens if you put out burning magnesium with liquid nitrogen! ❤️ ❤️ ❤️ Your support and encouragement inspire me to continue producing extraordinary chemical content! https://www.patreon.com/c/ChemicalForce ❤️ ❤️ ❤️

8Yh0dMZkHQ0 | 28 Jul 2025
Hey! In this episode I show you how potassium superoxide reacts with ozone to form bright red potassium ozonide KO3 - a highly reactive and unstable compound and what happens when liquid ozone meets KO₂ (spoiler: it EXPLODES 💥) ❤️ ❤️ ❤️ Your support inspire me to continue producing exotic chemical content! Patreon: https://www.patreon.com/c/ChemicalForce ❤️ ❤️ ❤️ Thank you!

tamZCtZysbQ | 30 Jun 2025
When heated, cobalt formate turns into a pyrophoric metal that can ignite spontaneously in air. I show how this reactive cobalt burns and how it instantly ignites when exposed to liquid oxygen. One of the coolest parts is the reaction between pyrophoric cobalt and liquid chlorine. This causes a violent reaction that produces beautiful blue smoke — anhydrous cobalt chloride. __________ ❤️ ❤️ ❤️ Your support inspire me to continue producing exotic chemical content! Patreon: https://www.patreon.com/c/ChemicalForce ❤️ ❤️ ❤️ __________

C6FHCbqUf3M | 28 May 2025
Hey! Watch what happens when non-self-igniting in air phosphine meets ozone. Spoiler: flasks were broken :D __________ 0:00 synthesis of aluminum phosphide (Al + P → AlP) 0:31 Formation of non-self-igniting phosphine by hydrolysis of aluminum phosphide 0:43 Phosphine and ozone reaction 2:42 Calcium carbide and water 3:07 Solid acetylene and ozone 5:30 Ozone solution in carbon tet 7:07 Carbide + water + liquid ozone = -flask :D __________ ❤️ ❤️ ❤️ https://www.patreon.com/c/ChemicalForce ❤️ ❤️ ❤️

9EVrIcaVrqM | 26 Mar 2025
In this video I convert oxygen into ozone using corona discharge and show experiments with liquid and gaseous ozone! __________ 0:00 What's inside my ozone machine 0:19 Ozone generation using ozone machine 1:57 Demonstration of ozone explosiveness from impact 3:25 Ignition of turpentine in ozone vapor 3:56 Obtaining pure liquid ozone 4:29 Demonstration that ozone is a pale blue gas 4:46 A very violent reaction between ozone and nitrogen monoxide 5:54 Oxidation of iodide ions by ozone to elemental iodine 6:40 Oxidation of iron ions (2+) by ozone to iron ions (3+) 7:08 Oxidation of manganese ions by ozone to permanganate ions 7:34 Oxidation of manganese ions by ozone to MnO2 precipitate 7:54 Formation of silver peroxide by the interaction of liquid ozone and silver nitrate solution 8:08 Mercury mirror 9:12 Gaseous ozone and silver 9:28 Gaseous ozone and Thallium 10:10 Anhydrous hydrazine and liquid ozone reaction! __________ ❤️ ❤️ ❤️ Your support inspire me to continue producing exotic chemical content! __________ Patreon: https://www.patreon.com/chemicalforce PayPal: @chemicalforce USDT(erc20): 0xD6609d8021138ddA718b01293FC94C9a61a2fdDa ❤️ ❤️ ❤️ __________ Thank you!

cGvhxfDFnTM | 06 Mar 2025
One of the earliest known explosive compounds left behind mysterious purple smoke and violet stains — an unsolved mystery for centuries. In this video, I’ll show you the explosive gold that alchemists discovered over 500 years ago, along with the mysterious purple smoke and stains it produces. https://patreon.com/ChemicalForce

83ayaVE1WgA | 17 Feb 2025
In this video I synthesized cyanogen (C2N2), a gas that has one of the highest combustion heats, the combustion temperature of cyanogen is more than 4000℃! Moreover, to increase the temperature, I added to the mixture not just oxygen, but its mixture with ozone! Having such a temperature I tried to melt tungsten! ❤️❤️❤️ https://www.patreon.com/c/ChemicalForce ❤️❤️❤️

0yxOTUtjB2U | 08 Jan 2025
Nitrosyl Chloride is a yellow gas that is commonly encountered as a component of aqua regia, a mixture of 3 parts concentrated hydrochloric acid and 1 part of concentrated nitric acid. It is a strong electrophile and oxidizing agent. Aqua regia (HNO3 + HCl) is well-known for its ability to dissolve gold. It's able to do this because it contains nitrosyl chloride - NOCl. In this video I synthesized Nitrosyl chloride and demonstrated its reaction with gold! _____ 0:00 - Formation of aqua regia (mixing nitric acid with hydrochloric acid) 0:35 - Synthesis of pure nitrosyl chloride 3:42 - NOCl Hydrolysis 4:05 - Chlorine vapors and acetylene 4:23 - Nitrosyl chloride and acetylene 4:37 - Burning magnesium in an nitrosyl chloride atmosphere 5:12 - Reaction with potassium iodide 5:42 - Reaction with Hydrogen sulfide 6:16 - Reaction with gold _____ Support me on Patreon! Thank you! https://www.patreon.com/ChemicalForce

9E4WQQk8p4k | 25 Dec 2024
In this video I synthesized three Uranium oxides (one of which - uranium dioxide is the main component of nuclear fuel) and mixed them with rocket fuel - Hydrazine! __________ 0:00 Intro 0:28 Radioactivity of uranyl nitrate 1:19 Uranyl nitrate glows under ultraviolet light 1:43 Fluorescence of aqueous solution UO2(NO3)2 2:06 Loss of fluorescence upon melting 2:25 Testing the fluorescence intensity of uranyl nitrate in liquid nitrogen 2:35 Complete decomposition of uranyl nitrate upon heating 3:06 Obtaining insoluble sodium salt! 3:36 Precipitation of uranium ferrocyanide 3:51 Formation of uranium tetrafluoride UF4 4:21 Uranium peroxide synthesis UO4 5:50 Uranium trioxide synthesis UO3 7:45 Uranium dioxide synthesis UO2 - nuclear fuel 8:51 I mix hydrazine with uranium oxides 10:45 Cool glow of uranyl nitrate formula :D __________ ❤️ https://www.patreon.com/ChemicalForce

jKf67grhc4w | 10 Dec 2024
Hey! In this video I show a little bit of the chemistry of Nitrogen Monoxide (NO). The main reaction of this video is the explosive interaction of nitrogen monoxide with ozone with the release of light (chemiluminescence)! ____________ 0:00 Intro 0:22 The explosive interaction of NO with O3 4:15 Oxidation of nitrogen monoxide in air 4:31 Condensation of nitric oxide 4:48 Liquid nitric oxide and liquid oxygen 5:10 Nitrous anhydride (or dinitrogen trioxide) demonstration 5:24 The true color of liquid nitrogen monoxide 6:22 Red dimer of nitric oxide 7:08 Qualitative reaction to nitrogen monoxide 7:34 Nitric oxide and iodine pentoxide 8:03 Reaction of nitrogen monoxide with hemoglobin ____________ ❤️❤️❤️ https://www.patreon.com/ChemicalForce ❤️❤️❤️

WDVf7ICcL7o | 25 Nov 2024
This video is about how I managed to dissolve a gold bar in a solution of potassium cyanide :D _______ 0:00 Intro 0:10 Potassium ferrocyanide drying 0:27 Potassium cyanide obtaining 2:12 Cyanide indicator paper preparing 3:05 Cyanide indicator paper demonstrating 3:35 Making a black penny (Copper coin experiment) 4:37 Dissolving gold foil in potassium cyanide solution 6:17 Dissolving gold ingot in potassium cyanide solution 8:54 Gold extraction with Zinc _______ Thank you for becoming my patrons, this is incredibly important to me due to the low number of views of my videos on YouTube! ❤️ ❤️ ❤️ Patreon: https://www.patreon.com/ChemicalForce

V6vURqcKc_0 | 31 Oct 2024
In this video I change the color of my blood by passing various gases through it such as carbon monoxide and hydrogen sulfide. __________ 0:00 - Intro 0:51 - Reaction of blood and carbon monoxide with the formation of carboxyhemoglobin 2:28 - Reaction of blood and oxygen with the formation of oxyhemoglobin 2:57 - Blood and Cyanide 3:42 - Comparison of shades of normal blood with carboxyhemoglobin and oxyhemoglobin 4:22 - Destruction of hemoglobin by hydrogen sulfide 6:15 - Comparing Shades of Blood __________ ❤️❤️❤️ https://www.patreon.com/ChemicalForce ❤️❤️❤️

90VUMPl15mc | 12 Oct 2024
Hello everyone! In this video I will show you the simplest in synthesis and interesting in reactions salt of nitrosyl - perchlorate NOClO4 ______________________________ 0:00 Intro 0:22 Synthesis 2:03 Decomposition upon heating 3:03 Hydrolysis 3:34 Reaction with ethanol 4:29 Reaction with acetone 5:08 Reaction with diethyl ether 5:55 Reaction with anhydrous hydrazine 6:15 Reaction with phenylhydrazine 7:10 Reaction with turpentine 7:25 Reaction with Borane-ammonia Complex 8:08 Outro ______________________________ ❤️ ❤️ ❤️ Your support and encouragement inspire me to continue producing extraordinary chemical content! __________ Patreon: https://www.patreon.com/ChemicalForce __________ ❤️ ❤️ ❤️

0jXcKaPnWvg | 13 Sep 2024
Earlier I made a video where I soaked burning magnesium into CCl4. In this video I dissolved ozone in tetrachloromethane and soaked burning magnesium into it. There is a difference! __________ ❤️ ❤️ ❤️ Your support and encouragement inspire me to continue producing extraordinary chemical content! __________ Patreon: https://www.patreon.com/ChemicalForce PayPal: @chemicalforce __________ crypto: BTC: bc1q2un9zn2gdvfjuguuujufm0qjvzumfpv7sj9xxq ------- USDT (ETH): 0xd709503113c1Ea2090858dB962E7970BB9aAF93d ❤️ ❤️ ❤️ __________

r6IFek6aefM | 19 Aug 2024
In this video, I experiment with Rupert drops and hydrofluoric acid to find out: Will a Rupert drop explode if its tail dissolves in this glass-dissolving acid? Watch to discover the surprising answer! __________ ❤️ ❤️ ❤️ Your support and encouragement inspire me to continue producing extraordinary chemical content! __________ Patreon: https://www.patreon.com/ChemicalForce PayPal: @chemicalforce __________ crypto:BTC: bc1q2un9zn2gdvfjuguuujufm0qjvzumfpv7sj9xxq ------- USDT (ETH): 0xd709503113c1Ea2090858dB962E7970BB9aAF93d ❤️ ❤️ ❤️ __________

fqXK4-Y1Tc0 | 23 Jul 2024
In this video we will continue experiments with vanadium compounds. This time I will show you reactions with liquid vanadium tetrachloride - VCl4. Reacting with moist air, crystals resembling flowers or cauliflower grow from this liquid! Enjoy! __________ 0:00 Vanadium and chlorine reaction 0:09 Presentation of ampoule with vanadium tetrachloride 0:38 Opening an ampoule with vanadium tetrachloride 1:42 Distillation of vanadium tetrachloride 2:20 Demonstration of pure vanadium tetrachloride 3:06 Crystal growth on the surface of vanadium tetrachloride 5:22 Demonstration of some chemical reactions using vanadium tetrachloride __________ ❤️ ❤️ ❤️ Your support and encouragement inspire me to continue producing extraordinary chemical content! __________ Patreon: https://www.patreon.com/ChemicalForce PayPal: @chemicalforce __________ crypto:BTC: bc1q2un9zn2gdvfjuguuujufm0qjvzumfpv7sj9xxq ------- USDT (ETH): 0xd709503113c1Ea2090858dB962E7970BB9aAF93d ❤️ ❤️ ❤️ __________

flvzaovQmVo | 02 Jul 2024
In this video I synthesized vanadium trichloride using disulfur dichloride, which in turn was synthesized from the elements: Sulfur and Chlorine. 2V2O5 + 6S2Cl2 = 4VCl3 + 5SO2 + 7S __________ 0:00 Synthesis of disulfur dichloride 2:19 S2Cl2 burning 3:04 Disulfur dichloride hydrolysis 3:20 Disulfur dichloride and nitric acid reaction 3:44 Dissolution of phosphorus in disulfur dichloride 4:09 I dip magnesium in disulfur dichloride 4:46 Synthesis of vanadium trichloride 6:46 Preparation of vanadium mirror 7:34 Multi-colored vanadium dichloride adducts 7:56 vanadium trichloride and anhydrous hydrazine __________ ❤️ ❤️ ❤️ Your support and encouragement inspire me to continue producing extraordinary chemical content! __________ Patreon: https://www.patreon.com/ChemicalForce PayPal: @chemicalforce __________ crypto:BTC: bc1pdhlsspcpmxl3sdcr4xanlc3qaz4p7njhqvpscxx4zphcfztkwtyq0gygvn ------- USDT (ETH): 0x2BbFD4aDEc7520301a408eBf7dD87B8b9935e49C ❤️ ❤️ ❤️

C7DCLi5I_nI | 03 Jun 2024
Hi! Tert-butyllithium (1.6M in pentane solution) is a pyrophoric liquid used in organic synthesis. It is highly flammable in air, so I added it to liquid oxygen! :D __________ 0:00 Carbon monoxide and liquid oxygen reaction 0:13 Burning sulfur and liquid oxygen reaction 1:04 Sulfur in carbon disulfide solution demonstration 1:42 Sulfur in carbon disulfide solution and liquid oxygen reaction 2:38 Lithium borohydride combustion in air = RED color 3:01 Lithium borohydride combustion in liquid oxygen = GREEN color 3:20 Trimethyl borate 4:13 Decaborane in trimethyl borate solution 5:12 tert-Butyllithium solution unpacking 6:36 tert-Butyllithium spontaneous combustion 7:07 tert-Butyllithium spontaneously ignites in ozonated oxygen 7:33 I add tert-Butyllithium drop by drop into liquid oxygen 9:02 I pour a stream of tert-butyllithium into liquid oxygen 10:07 tert-Butyllithium and liquid chlorine reaction! 10:45 Incredible shot 11:00 I LOVE MY PATRONS __________ ❤️ ❤️ ❤️ Your support and encouragement inspire me to continue producing extraordinary chemical content! __________ Patreon: http://patreon.com/chemicalforce PayPal: @chemicalforce __________ crypto:BTC: bc1pdhlsspcpmxl3sdcr4xanlc3qaz4p7njhqvpscxx4zphcfztkwtyq0gygvn ------- USDT (ETH): 0x2BbFD4aDEc7520301a408eBf7dD87B8b9935e49C ❤️ ❤️ ❤️

fQyjz86GBcE | 13 May 2024
A few years ago Tom (ExF) and I did a collaboration and he showed the spontaneous combustion of silane and diborane. I went further and in this video I'll show you in addition to silane and diborane also phosphine, titanium tetrahydride and possibly stibine and stannane. I cannot confirm the formation of the last two gases so see for yourself and leave comments. __________ 0:00 Lithium hydride demonstration 0:51 Lithium hydride reacts with water and producing a huge amount of hydrogen. 1:02 Burning of lithium hydride 1:30 Lithium hydride melting 2:04 Lithium hydride produces Phosphine 3:12 Silicon tetrachloride demonstration 3:46 Lithium hydride produces Silane 5:00 Boron tribromide demonstration BBr3 5:15 Lithium hydride produces Diborane 6:40 Antimony pentachloride demonstration SbCl5 6:53 Lithium hydride produces Stibine (???) 8:55 Titanium tetrachloride demonstration TiCl4 9:00 Lithium hydride produces Titanium tetrahydride 10:21 Lithium hydride produces Stannane (???) __________ ❤️ ❤️ ❤️ Your support and encouragement inspire me to continue producing extraordinary chemical content! __________ Patreon: https://www.patreon.com/ChemicalForce PayPal: @chemicalforce __________ crypto:BTC: bc1pdhlsspcpmxl3sdcr4xanlc3qaz4p7njhqvpscxx4zphcfztkwtyq0gygvn ------- USDT (ETH): 0x2BbFD4aDEc7520301a408eBf7dD87B8b9935e49C ❤️ ❤️ ❤️
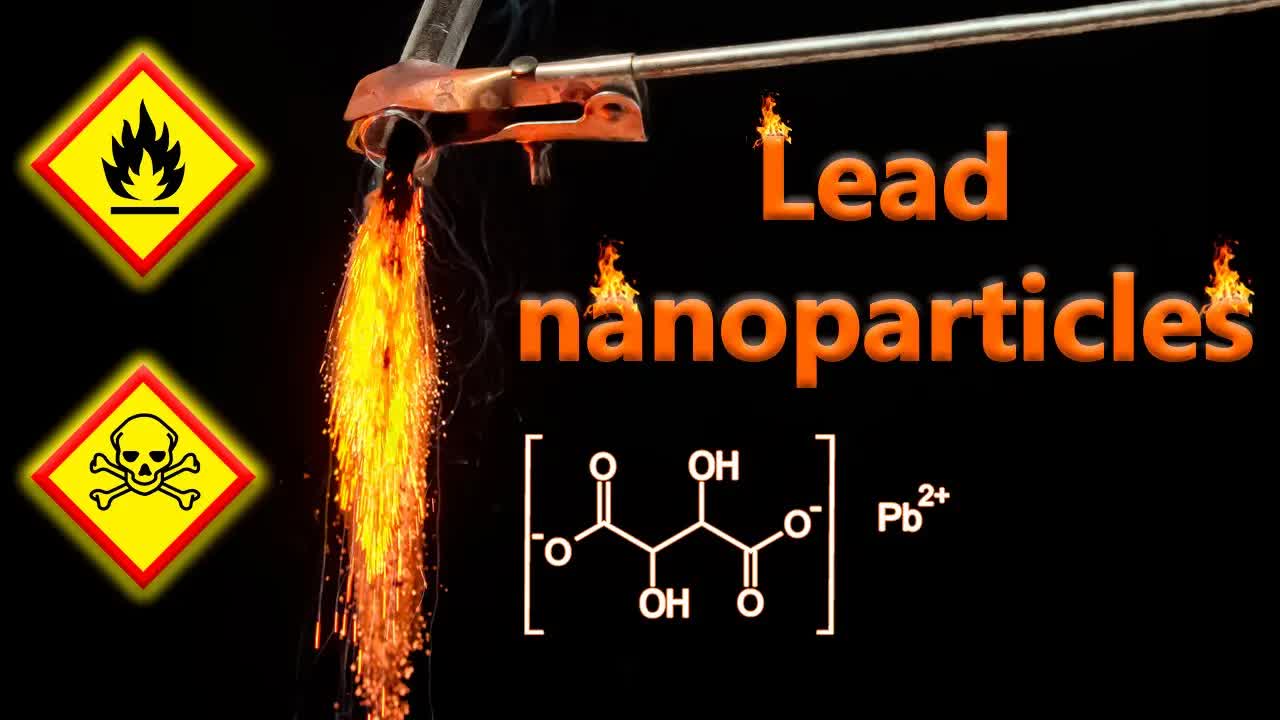
X4dt1lRBMrc | 30 Apr 2024
In this video I'll show you an exciting experiment with lead tartrate. By heating this compound in a test tube sealed with cotton wool to limit air exposure, we observe how lead tartrate decomposes into lead nanoparticles which ignite spontaneously when exposed to air! _________ 0:00 Lead tartrate synthesis 0:23 Lead tartrate decomposition 0:53 Combustion of lead nanoparticles in air 2:50 Combustion of lead nanoparticles in oxygen atmosphere 4:03 Combustion of lead nanoparticles in liquid oxygen 5:11 Adding liquid oxygen to pyrophoric lead nanoparticles 6:51 Combustion of lead nanoparticles in chlorine atmosphere 7:31 Combustion of lead nanoparticles in liquid chlorine 8:15 Adding pyrophoric lead to heated iodine 9:10 Adding pyrophoric lead to fuming nitric acid _________ ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: @chemicalforce __________ crypto:BTC: bc1pdhlsspcpmxl3sdcr4xanlc3qaz4p7njhqvpscxx4zphcfztkwtyq0gygvn ------- USDT (ETH): 0x2BbFD4aDEc7520301a408eBf7dD87B8b9935e49C

EDfGaq_X0cw | 22 Apr 2024
SeOCl2 - Selenium oxychloride. It's closest relative of thionyl chloride. This liquid has better dissolving ability towards most non-metals and other substances. In this video I'll show the synthesis and chemical properties of selenium oxydichloride. ____________________ 0:00 Selenium oxydichloride preparatory stage 0:51 Selenium oxydichloride synthesis 3:11 Vacuum distillation of SeOCl2 4:08 Pure seleninyl chloride demonstration 4:48 Selenium dissolution 5:17 Ignition of phosphorus instead of dissolution 6:21 Dissolution of Arsenic, Iodine, Antimony, Sulfur 6:41 Thionyl chloride and seleninyl chloride comparison of chemical properties 13:05 Reaction between seleninyl chloride and potassium 13:38 Tellurium and fly :D ____________________ ❤️ ❤️ ❤️ Your support and encouragement inspire me to continue producing exotic chemical content! __________ Patreon: https://www.patreon.com/ChemicalForce PayPal: @chemicalforce __________ crypto:BTC: bc1pdhlsspcpmxl3sdcr4xanlc3qaz4p7njhqvpscxx4zphcfztkwtyq0gygvn ------- USDT (ETH): 0x2BbFD4aDEc7520301a408eBf7dD87B8b9935e49C ❤️ ❤️ ❤️ __________

jVBFYM58M-0 | 11 Apr 2024
Hey! In this video I'll show you liquefied carbon monoxide. I’ll show you how it burns, how it interacts with liquid oxygen and nitrous oxide, in general... as usual a bunch of experiments that you’re unlikely to see anywhere else :D __________ 0:00 Burning carbon monoxide in air 0:15 Burning carbon monoxide in oxygen 0:55 Liquid carbon monoxide demonstration 3:07 I pour burning carbon monoxide on the table 4:08 I pour burning carbon monoxide into liquid oxygen 6:05 Will-o'-the-wisp 7:39 I pour burning carbon monoxide into liquid nitrous oxide 9:15 Qualitative reaction to carbon monoxide with iodine pentoxide 10:32 Palladium ions are sensitive to carbon monoxide 11:27 Tollens' reagent and carbon monoxide 11:50 Сhromium hexacarbonyl decomposition by nitric acid 12:15 Interaction between carbon monoxide and nitrogen dioxide 12:53 I add burning carbon monoxide to nitrogen tetroxide 13:20 Does manganese heptoxide ignite liquid carbon monoxide on contact? 13:47 end credits __________ ❤️ ❤️ ❤️ Your support and encouragement inspire me to continue producing extraordinary chemical content! __________ Patreon: https://www.patreon.com/ChemicalForce PayPal: @chemicalforce __________ crypto:BTC: bc1pdhlsspcpmxl3sdcr4xanlc3qaz4p7njhqvpscxx4zphcfztkwtyq0gygvn ------- USDT (ETH): 0x2BbFD4aDEc7520301a408eBf7dD87B8b9935e49C ❤️ ❤️ ❤️ __________

gs0V4WTSc7g | 01 Apr 2024
Hey! In this video I give injections of alkali alloy into various fruits :D ______ 0:00 Intro 0:25 Tangerine 🍊 1:00 Grapefruit 1:27 Tomato 🍅 2:01 Cucumber 🥒 2:25 Apple 🍏 3:07 Banana 🍌 3:42 Kiwi 🥝 4:20 Coconut 🥥 5:16 Onion 🧅 6:44 Lemon 🍋 7:24 Lime 7:55 Pomegranate 8:24 Plum 8:58 Fig 9:31 Red bell papper 🫑 ______ ❤️ ❤️ ❤️ Your support and encouragement inspire me to continue producing exotic chemical content! __________ Patreon: https://www.patreon.com/ChemicalForce PayPal: @chemicalforce __________ crypto:BTC: bc1pdhlsspcpmxl3sdcr4xanlc3qaz4p7njhqvpscxx4zphcfztkwtyq0gygvn ------- USDT (ETH): 0x2BbFD4aDEc7520301a408eBf7dD87B8b9935e49C ❤️ ❤️ ❤️ __________

XE2dkgWyXvs | 26 Mar 2024
Hi! This alloy is very similar in properties to the rubidium-cesium alloy, but I decided to make this video anyway to showcase the most fusible alloy in the world. _____ 0:00 Intro 1:26 Preparation of the world's most fusible alloy 2:10 Squeezing from a syringe 2:44 Freezing the alloy 4:22 Reaction with ice 5:18 Reaction with liquid oxygen 6:36 Reaction with liquid chlorine 8:14 Reaction with nitrogen tetroxide (liquid nitrogen dioxide) 8:32 Injection into tomato 9:18 Injection into tangerine 10:00 Reaction with fluorosulfonic acid HSO3F _____ __________ ❤️ ❤️ ❤️ Your support and encouragement inspire me to continue producing extraordinary chemical content! __________ Patreon: / chemicalforce PayPal: @chemicalforce __________ crypto:BTC: bc1pdhlsspcpmxl3sdcr4xanlc3qaz4p7njhqvpscxx4zphcfztkwtyq0gygvn ------- USDT (ETH): 0x2BbFD4aDEc7520301a408eBf7dD87B8b9935e49C ❤️ ❤️ ❤️ __________

bEMGr5i3uGs | 13 Mar 2024
Hi! We have all seen the video about sodium-potassium alloy (NaK) many times. In this video, you will discover the chemical properties and reactions of a much more active alloy: rubidium and cesium (RbCs). __________ 0:00 Intro 1:08 Preparation of rubidium cesium alloy 2:32 I squeeze the alloy out of the syringe 5:33 Rubidium Cesium alloy and oxygen 8:25 Rubidium Cesium alloy reacts with water 8:56 Tomato RbCs-injection 9:36 Rubidium-Cesium alloy and liquid chlorine 10:52 Rubidium-Cesium alloy and bromine 11:40 Rubidium-Cesium alloy and iodine 13:46 Rubidium-Cesium alloy and sulfur 15:12 Rubidium-Cesium alloy and sulfuric acid H2SO4 15:33 Rubidium-Cesium alloy and selenium 16:14 Rubidium-Cesium alloy and selenic acid H2SeO4 16:39 Rubidium-Cesium alloy and tellurium 18:04 Rubidium-Cesium alloy and anhydrous hydrazine __________ ❤️ ❤️ ❤️ Your support and encouragement inspire me to continue producing extraordinary chemical content! __________ Patreon: https://www.patreon.com/ChemicalForce PayPal: @chemicalforce __________ crypto:BTC: bc1pdhlsspcpmxl3sdcr4xanlc3qaz4p7njhqvpscxx4zphcfztkwtyq0gygvn ------- USDT (ETH): 0x2BbFD4aDEc7520301a408eBf7dD87B8b9935e49C ❤️ ❤️ ❤️ __________

-kfrIUJFguc | 07 Mar 2024
Hi! While I'm working on a more interesting video about alkali metals, I decided to edit a simple video about the reaction of anhydrous hydrazine with copper compounds. Enjoy! __________ ❤️ ❤️ ❤️ Your support and encouragement inspire me to continue producing extraordinary content! __________ Patreon: https://www.patreon.com/ChemicalForce PayPal: @chemicalforce __________ crypto:BTC: bc1pdhlsspcpmxl3sdcr4xanlc3qaz4p7njhqvpscxx4zphcfztkwtyq0gygvn ------- USDT (ETH): 0x2BbFD4aDEc7520301a408eBf7dD87B8b9935e49C ❤️ ❤️ ❤️ __________

8Pkisj2A0s0 | 22 Feb 2024
Hi! This is a continuation of the video about the heaviest gas in the world - tungsten hexafluoride (WF6). In this video, you will witness some chemical reactions involving it and also see what this gas looks like in a liquid state! __________ 0:00 Intro 0:33 I release the gas from the cylinder 1:02 Reaction of lead nitrate with hydrogen fluoride 1:47 Reaction of tungsten hexafluoride with water (WF6 + H2O) 2:28 The halide exchange reaction between tungsten hexafluoride and titanium tetrachloride (WF6 + TiCl4) 2:56 Tungsten blue formation 3:23 Alkali metal borohydrides and tungsten hexafluoride reactions 4:03 Sodium fluoride absorbs tungsten hexafluoride and forms double fluoride (2NaF + WF6 = Na2WF8) 4:50 Colored adducts formation between WF6 and organic substances 5:13 I aimed a stream of WF6 at the burning magnesium (WF6 + Mg) 5:32 Vigorous reaction with ammonia (WF6 + NH3) 6:57 Liquefied heaviest gas! 9:17 Liquid tungsten hexafluoride + Liquid ammonia 10:18 Liquid tungsten hexafluoride + Anhydrous Hydrazine 11:20 Reaction with borazane 12:00 Reaction with Lithium aluminum hydride __________ ❤️ ❤️ ❤️ Your support and encouragement inspire me to continue producing extraordinary content! __________ Patreon: https://www.patreon.com/ChemicalForce PayPal: @chemicalforce __________ crypto:BTC: bc1pdhlsspcpmxl3sdcr4xanlc3qaz4p7njhqvpscxx4zphcfztkwtyq0gygvn ------- USDT (ETH): 0x2BbFD4aDEc7520301a408eBf7dD87B8b9935e49C ❤️ ❤️ ❤️ __________

AtL2XlWTr3w | 17 Jan 2024
Hey! This video is about hydrogen telluride and its photodissociation. It was a very long and arduous synthesis, but I managed to obtain several milliliters of liquid H2Te. Regarding the smell, I wouldn’t say that its smell is similar to garlic, more like that of a just burnt match head. However, I can’t be sure about this; perhaps in high concentrations there is a similarity to the garlic smell. The main thing is that after this synthesis I don’t smell anything special :D _____ 0:00 Tellurium demonstration 0:29 Formation of zinc telluride from simple elements 0:55 Formation of aluminum telluride from simple elements 1:10 Demonstration of zinc telluride 1:26 Synthesis of hydrogen telluride from zinc telluride and hydrochloric acid 1:58 Hydrogen telluride combustion 2:44 Tellurium plasma 3:14 Combustion of hydrogen telluride in oxygen 4:05 Some chemical reactions with hydrogen telluride 6:05 Preparation of liquid hydrogen telluride and chemical reactions with it _____ To support me: ❤❤❤ https://www.patreon.com/ChemicalForce ❤❤❤ Crypto: BTC: bc1q2un9zn2gdvfjuguuujufm0qjvzumfpv7sj9xxq ETH, USDT (ERC20): 0xd709503113c1Ea2090858dB962E7970BB9aAF93d ❤❤❤

DnOQsC9MGbc | 21 Dec 2023
In this video I show the most destructive chemical reaction I have ever encountered in my chemical practice. Interestingly, this reaction is not described in any article. Please note that the reaction involves two non-explosive substances, however the product of this chemical reaction is incredibly explosive! Destruction Unleashed: Combining Decaborane and RFNA! __________ 0:00 Intro 0:37 Fuming nitric acid presentation 1:56 Decaborane presentation 3:00 Metal bowl destruction 3:54 Cardboard destruction! 4:35 Brick destruction! 5:21 Chemical card trick :D 5:58 Peach destruction 6:40 Baseball destruction 7:53 Watermelon destruction __________ ❤️ ❤️ ❤️ Your support and encouragement inspire me to continue producing extraordinary content! __________ Patreon: https://www.patreon.com/ChemicalForce PayPal: @chemicalforce __________ crypto: BTC: bc1pdhlsspcpmxl3sdcr4xanlc3qaz4p7njhqvpscxx4zphcfztkwtyq0gygvn ------- USDT (ETH): 0x2BbFD4aDEc7520301a408eBf7dD87B8b9935e49C ❤️ ❤️ ❤️

7dQtKNKMsCE | 15 Dec 2023
I upgraded the typical piranha solution by replacing sulfuric acid with selenic acid! H2SeO4 + H2O2 60% and compared their properties! ❤️ ❤️ ❤️ ❤️ ❤️ ❤️ https://www.patreon.com/ChemicalForce ❤️ ❤️ ❤️ ❤️ ❤️ ❤️

xGpm_kAlnFo | 30 Nov 2023
Tungsten hexafluoride is the heaviest gas in the world. It is approximately 11 times heavier than air! This video mostly talks about the physical properties of the world's heaviest gas. I decided to show its chemical properties in one of my next videos, so be sure to subscribe to the channel so you don't miss out! :D Enjoy watching! ____________________ 0:00 Intro 0:12 I release tungsten hexafluoride from a cylinder 0:43 The world's heaviest gas VS flowers! 2:02 Tungsten hexafluoride VS arugula 2:58 The heaviest gas in the world VS my hair 3:24 Industrial application of tungsten hexafluoride 6:00 Demonstration of the density of tungsten hexafluoride 8:31 My voice in an atmosphere of tungsten hexafluoride 9:46 Inflating balloons with tungsten hexafluoride and playing with them! ____________________ ❤️ ❤️ ❤️ Your support and encouragement inspire me to continue producing extraordinary content! __________ Patreon: https://www.patreon.com/ChemicalForce __________ PayPal: @chemicalforce USDT (ETH): 0x2BbFD4aDEc7520301a408eBf7dD87B8b9935e49C ❤️ ❤️ ❤️

BJlnkUbtdhc | 23 Oct 2023
Hi all! Soon I'll be posting a video about the world's heaviest gas - Tungsten Hexafluoride WF6. Acquiring this substance was a significant challenge! 😎 The process of working on this video has been time-consuming (and continues to take up a lot of my time) 🕔, which is why I haven't published anything this month. I hope you understand that creating such videos requires a lot of resources. Thank you for your patience! 🤗 __________ Patreon: https://www.patreon.com/ChemicalForce __________ PayPal: @chemicalforce USDT (ETH): 0x2BbFD4aDEc7520301a408eBf7dD87B8b9935e49C

drQQ41st7hs | 18 Sep 2023
I managed to capture a very interesting, never-before-described chemical reaction between potassium superoxide and phosphorus chlorides. Discover the astonishing formation of fireblobs that occur when two typically non-flammable chemicals come into contact and ignite in a mesmerizing chemical reaction. ================= 0:00 Intro 0:23 Liquid chlorine and Phosphorus reaction 1:22 Phosphorus trichloride demonstration 2:34 Phosphorus pentachloride demonstration 3:03 Liqiod Oxygen and Liquid Potassium demonstration 3:14 Explosive reactions with potassium superoxide 5:03 Fireblobs Formation ================= ❤️ ❤️ ❤️ Your support and encouragement inspire me to continue producing extraordinary content! __________ Patreon: https://www.patreon.com/ChemicalForce __________ PayPal: @chemicalforce USDT (ETH): 0x2BbFD4aDEc7520301a408eBf7dD87B8b9935e49C ❤️ ❤️ ❤️

llvRHCqWlQw | 06 Sep 2023
I obtained tungsten mirrors by the decomposition of W(CO)6 (tungsten hexacarbonyl) in an inert gas atmosphere and the reaction of vapors of WCl6 (tungsten hexachloride) and WF6(tungsten hexafluoride) with hydrogen. This is the first video on YouTube where you can see the heaviest gas in the world: Tungsten Hexafluoride! A separate video about this substance will be later, don't miss it! ================= 0:00 tungsten mirrors demonstrations 0:42 Obtaining tungsten from tungsten hexacarbonyl 2:21 Some reactions with tungsten hexacarbonyl 3:07 Tungsten hexachloride ampoule presentation 3:32 Tungsten hexachloride and liquid nitrogen 4:07 WCl6 chemical properties (including reaction with hydrazine) 5:34 Obtaining tungsten from tungsten hexachloride 9:10 Obtaining tungsten from tungsten hexafluoride WF6 ================= ❤️ ❤️ ❤️ Your support and encouragement inspire me to continue producing extraordinary content! __________ Patreon: https://www.patreon.com/ChemicalForce __________ PayPal: @chemicalforce USDT (ETH): 0x2BbFD4aDEc7520301a408eBf7dD87B8b9935e49C ❤️ ❤️ ❤️

PxH8_ZGMG5E | 12 Aug 2023
In this video, I want to clarify that I'm NOT PROMOTING Absolut vodka; I'm simply using one of the most recognizable vodka brands. It was a challenging endeavor, especially in terms of proper lighting setup within the fume hood. 🥲 Unfortunately, it's important to note that the results with vodka weren't as successful. As a result, for the reaction, I use ethyl alcohol with a water content of up to 10%, or vodka with an alcohol content of at least 90% 😀 Nevertheless the formula (C2H5OH + H2O) is correct, it just doesn't reveal the ratio of the ingredients 😂 ❤️❤️❤️❤️❤️❤️❤️❤️❤️❤️❤️❤️❤️❤️❤️❤️ Patreon: https://www.patreon.com/ChemicalForce PayPal: @chemicalforce ❤️❤️❤️❤️❤️❤️❤️❤️❤️❤️❤️❤️❤️❤️❤️❤️

cjMZV2d1HIw | 24 Jul 2023
*In all reactions where "H2SeO4 conc." means selenic acid obtained after ordinary distillation with a concentration of about 85%. Welcome to our captivating journey into the fascinating world of chemistry! In this exciting new video, we explore the incredible properties of Selenic Acid, a powerful and unique chemical compound. Prepare to be amazed as I demonstrate the process of obtaining pure, crystalline Selenic Acid through meticulous vacuum distillation. Witness the magic of frozen seed crystals that bring this chemical to life in breathtaking forms! 😅 Watch as gold and silver dissolve in this extraordinary acid. Hold your breath as we ignite an explosive encounter between Selenic Acid and Anhydrous Hydrazine 🤯 ============================= 0:00 Selenium dioxide synthesis from selenium and oxygen. 0:49 Selinous acid synthesis. 1:46 Selenic acid synthesis. 2:18 Anhydrous selenic acid synthesis (vacuum distillation). 5:09 Crystalline selenic acid synthesis. 8:13 Dissolving of gold in selenic acid. 9:51 Dissolving of silver in selenic acid. 10:35 Molten selenic acid burns through paper. 10:50 Sulfuric acid and sugar. 11:22 Selenic acid and sugar. 13:07 Thermal effect when selenic acid is dissolved in water. 13:39 The difference between selenic acid and sulfuric acid. 18:47 Explosive reaction between molten selenic acid and phosphorus. 20:11 Selenic acid crystalls and Anhydrous Hydrazine ============================= ❤️ ❤️ ❤️ Your support and encouragement inspire me to continue producing extraordinary content! __________ Patreon: https://www.patreon.com/ChemicalForce PayPal: @chemicalforce __________ crypto: BTC: bc1pdhlsspcpmxl3sdcr4xanlc3qaz4p7njhqvpscxx4zphcfztkwtyq0gygvn ------- USDT (ETH): 0x2BbFD4aDEc7520301a408eBf7dD87B8b9935e49C ❤️ ❤️ ❤️

BsO-tV2XK8M | 26 Jun 2023
Hey! One of My next videos will be about selenic acid H2SeO4, but for now I have prepared reactions with elemental selenium for you. While you're watching this video, I'm trying to get rid of the smell of garlic :D ______________________________________________ 0:00 Intro 0:32 Selenium demonstration 1:50 Selenium plasma 2:53 Formation of selenium granules 5:42 Plastic selenium 6:15 Red selenium synthesis 9:25 Combustion of selenium in pure oxygen 10:15 Selenium dioxide and its reaction with anhydrous hydrazine 13:02 Selenium and nitric/sulfuric/iodic acid reactions 14:25 Selenium and alkali metals 15:41 Qualitative reaction to hydrogen selenide 16:34 Selenium tetrachloride ______________________________________________ ❤️ 💛 💚 If you enjoy what I do and would like to help me to create unique chemical content please support me on Patreon. __________ Patreon: https://www.patreon.com/ChemicalForce PayPal: @chemicalforce __________ crypto: BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 USDT (ETH): 0x2BbFD4aDEc7520301a408eBf7dD87B8b9935e49C ❤️ 💛 💚

x8yQAm1ua7s | 27 May 2023
Hey! In this short video I demonstrate some reactions of sodium-potassium alloy with halogens: liquid chlorine, bromine, iodine. Enjoy! I apologize for not including the names of my patrons in the credits., so I post them here. I love all my patrons no matter the tier! ❤️ Franek Kilarski ❤️ BlockChain Omega ❤️ nick ❤️ Kenyaa ❤️ Brant Longest ❤️ Brian Peznowski ❤️ Andis ❤️ Sir Wuffleton ❤️ SlcBill ❤️ André Forcier ❤️ sabrina ❤️ Shane Wallerick ❤️ JH ❤️ TimeO ❤️ Catherine ❤️ Robert Kupper ❤️ andrew dorsey ❤️ Mark Jobes ❤️ John Hamberger jn. ❤️ The Gayest Person on Patreon ❤️ Dahan ❤️ Sebastian Dörr ❤️ Stan Presolski ❤️ Duncan Wilkie ❤️ Jack Hopper ❤️ Aron Somodi ❤️ Dhruv Singhal ❤️ Gabry ❤️ William Ching ❤️ Riley Reprogle ❤️ ioPhileas ❤️ Ilikeplants andgrowingthings ❤️ Acronus ❤️ Wesley Gardner ❤️ Cristian Smith ❤️ John Libal ❤️ _____ ❤️ 💛 💚 If you enjoy what I do and would like to help me to create unique chemical content please support me on Patreon. __________ Patreon: https://www.patreon.com/ChemicalForce PayPal: @chemicalforce __________ crypto: BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 USDT (ETH): 0x2BbFD4aDEc7520301a408eBf7dD87B8b9935e49C ❤️ 💛 💚

VUfJWuhmygA | 22 May 2023
The inspiration for this video came from an article in the well-known journal, Applied Chemistry. https://doi.org/10.1002/anie.201605986 In short, we can observe the blue color of solvated electrons not only when alkali metals are dissolved in liquid ammonia but also in water! http://jungwirth.uochb.cas.cz/assets/papers/paper278.pdf _____ 0:00 Sodium-potassium alloy making 3:00 Solvated electrons in liquid ammonia 3:45 NaK and water 4:20 Solvated electrons in water 5:56 I Light a Match with Water! 6:33 Superheated steam and Lithium aluminum hydride 7:17 Superheated steam and sodium-potassium alloy _____ ❤️ 💛 💚 If you enjoy what I do and would like to help me to create unique chemical content please support me on Patreon. __________ Patreon: https://www.patreon.com/ChemicalForce PayPal: @chemicalforce __________ crypto: BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 USDT (ETH): 0x2BbFD4aDEc7520301a408eBf7dD87B8b9935e49C ❤️ 💛 💚

7wxRbr-beK4 | 24 Apr 2023
In fact in this video I just wanted to show you the incredibly beautiful reactions between titanium and iodine in slowmo, but later I decided to increase the duration of the video a little and added some footages of titanium tetraiodide (TiI4) to it. Enjoy! ❤️ 💛 💚 If you enjoy what I do and would like to help me to create unique chemical content please support me on Patreon! __________ Patreon: https://www.patreon.com/ChemicalForce PayPal: @chemicalforce __________ crypto: BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ❤️ 💛 💚

vHepnY2CNXA | 30 Mar 2023
This is a dangerous explosive version on a classic “Barking dog” chemical reaction! ❤️ 💛 💚 If you enjoy what I do and would like to help me to create unique chemical content please support me on Patreon. __________ Patreon: https://www.patreon.com/ChemicalForce PayPal: @chemicalforce __________ crypto: BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 USDT (ETH): 0x2BbFD4aDEc7520301a408eBf7dD87B8b9935e49C ❤️ 💛 💚

_9PkKB_7KyU | 17 Mar 2023
In this fascinating video, we delve into the world of hydrogen sulfide - a colorless, toxic gas with a distinct and unpleasant odor, often described as smelling like rotten eggs or fart. We start by synthesizing liquid hydrogen sulfide in the lab, using the hydrolysis of aluminum sulfide. You'll witness the chemical reaction taking place, producing high-purity hydrogen sulfide. As we explore the properties of liquid hydrogen sulfide, you'll discover that it is highly flammable and reacts explosively with some substances, such as chromyl chloride and dinitrogen tetroxide and you'll see sulfur forming in various reactions. We'll also examine the effects of bubbling hydrogen sulfide through concentrated nitric acid, resulting in the formation of large amounts of nitrogen dioxide, and how hydrogen sulfide reacts with iodine monochloride, producing elemental iodine. You'll also see some stunning reactions with other substances, such as lead dioxide, sodium and potassium peroxides, selenous acid, etc. ========================= 0:00 Making aluminum sulfide 1:23 Synthesis of liquid hydrogen sulfide 2:07 Common green bottle flies arrived in the lab during synthesis 2:25 Burning hydrogen sulfide 3:52 Drawing with hydrogen sulfide 5:13 Hydrogen sulfide and nitric acid/dinitrogen tetroxide 6:50 Hydrogen sulfide and osmium tetroxide 8:15 Hydrogen sulfide and chromyl chloride 9:19 Hydrogen sulfide and iodine monochloride 10:21 Ignition of liquid hydrogen sulfide upon contact with various oxidizers 12:59 Reactions with precipitation at a distance 14:31 The formation of two elements in the reaction of selenous acid and hydrogen sulfide 14:52 Beautiful reducing of cobalt trifluoride 15:57 Try to answer a chemistry question :D ========================= ❤️ 💛 💚 If you enjoy what I do and would like to help me to create unique chemical content please support me on Patreon 🥰 __________ Patreon: https://www.patreon.com/ChemicalForce PayPal: @chemicalforce Revolut: @chemicalforce __________ crypto: BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2Ethereum Tether (ETH) 0x2BbFD4aDEc7520301a408eBf7dD87B8b9935e49C ❤️ 💛 💚

7_Q568_tgik | 22 Feb 2023
In this video, you'll witness the mesmerizing beauty of chemical serpents, a fascinating and visually stunning chemical reaction. I'll show you the slow-motion decomposition of mercury thiocyanate, also known as "the pharaoh's serpent". We'll also experiment with adding different elements to the reaction, such as liquid oxygen, nitrous oxide, and liquid chlorine, to see how they affect the combustion and growth of the serpent. Additionally, I'll showcase a combo reaction of mercury thiocyanate decomposition and ammonium dichromate volcano, both in real time and slow motion. You'll see how these two reactions complement each other. And lastly, I'll introduce you to another chemical serpent that emerges when a mixture of p-nitroaniline and concentrated sulfuric acid is heated. So join me for a scientific journey into the world of chemical serpents and don't forget to like, comment, and subscribe to stay up to date with my latest chemical experiments! ========================= 0:00 Mercury thiocyanate decomposition 2:05 I add liquid oxygen to decomposing mercury thiocyanate 2:30 I add liquid nitrous oxide to decomposing mercury thiocyanate 3:03 I add liquid chlorine to decomposing mercury thiocyanate 3:44 Combo reaction of mercury thiocyanate decomposition and ammonium dichromate volcano 5:24 Catalytic oxidation of ammonia by oxygen 6:02 p-nitroaniline serpent ========================= Mechanism of polymerization of 4-nitroaniline: http://scienceline.ucsb.edu/getkey.php?key=3329 ========================= ❤️ 💛 💚 If you enjoy what I do and would like to help me to create unique chemical content please support me on Patreon 🥰 __________ Patreon: https://www.patreon.com/ChemicalForce PayPal: @chemicalforce Revolut: @chemicalforce __________ crypto: BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2Ethereum Tether (ETH) 0x2BbFD4aDEc7520301a408eBf7dD87B8b9935e49C ❤️ 💛 💚

tgYv7pb29yw | 03 Feb 2023
Join us on a journey of fire and chemistry as we explore the explosive reactions of burning magnesium with various liquid inorganic chlorides. Watch as the magnesium reacts with tin tetrachloride, silicon tetrachloride, germanium tetrachloride, and more to create powerful and visually stunning displays of energy. See how each reaction differs in intensity, brightness, and outcome. ____________________ 0:00 Intro 0:36 Burning magnesium and tin tetrachloride SnCl4 1:30 Burning magnesium and silicon tetrachloride SiCl4 2:34 Burning magnesium and germanium tetrachloride GeCl4 2:57 Burning magnesium and phosphorus trichloride PCl3 3:57 Burning magnesium and antimony pentachloride SbCl5 4::51 Burning magnesium and titanium tetrachloride TiCl4 5:11 Burning magnesium and oxalyl chloride (COCl)2 6:02 Burning magnesium and sulfuryl chloride SO2Cl2 6:43 Burning magnesium and thinoyl chloride SOCl2 ____________________ 🧡🧡🧡🧡🧡 If you enjoy what I do and would like to help me to create unique chemical content please support me on Patreon.😇 __________ Patreon: https://www.patreon.com/ChemicalForce PayPal: @chemicalforce Revolut: @chemicalforce __________ crypto: BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2Ethereum 🧡🧡🧡🧡🧡

JfQAi4Z-QqI | 24 Jan 2023
Today we delve into the world of vanadium chemistry and demonstrate the synthesis of vanadium oxytrichloride - yellow liquid that fumes in air with red-orange smoke. You'll also learn about the properties and reactions of vanadium oxytrichloride and dioxovanadium(V)chloride. ____________________ 0:00 Intro 0:30 Vanadium oxytrichloride synthesis 3:05 Vanadium oxytrichloride properties 4:28 Vanadium oxytrichloride and water reaction 5:22 Vanadium oxytrichloride and alkali solutions reactoins 6:38 The "chameleon" reaction, which is characteristic only for vanadium compounds 7:28 Vanadium oxytrichloride and alkali metals 9:17 I soak burning magnesium into VOCl3 9:59 Dioxovanadium (V) chloride synthesis: VO2Cl 12:16 Words of gratitude to my viewers and especially patrons 🧡 ____________________ 🧡🧡🧡🧡🧡 If you enjoy what I do and would like to help me to create unique chemical content please support me on Patreon 🥺 __________ Patreon: https://www.patreon.com/ChemicalForce PayPal: @chemicalforce Revolut: @chemicalforce __________ crypto: BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2Ethereum 🧡🧡🧡🧡🧡

V3HuKQvRBUE | 19 Dec 2022
Anhydrous hydrazine is a chemical compound with powerful reactive properties that make it an essential component in various industries, including aerospace and medicine. It is commonly used as a high-energy fuel in rocket propulsion, due to its ability to produce large amounts of energy through exothermic reactions. However, anhydrous hydrazine is also highly toxic and flammable, making it extremely dangerous to handle and use. In this video, we will explore the unique characteristics of anhydrous hydrazine, its uses and applications, and the precautions that must be taken when working with this powerful but potentially hazardous chemical. So, be careful when handling anhydrous hydrazine and always follow proper safety protocols. 0:00 Intro 0:45 Anhydrous hydrazine and oxygen 1:27 Anhydrous hydrazine and ozonated oxygen 1:53 Anhydrous hydrazine and Nitrous oxide 4:02 Just Me :D 4:26 Anhydrous hydrazine and Chromyl chloride 6:25 Liquid nitrogen dioxide presentation 6:54 Hypergolic reaction between Hydrazine and Dinitrogen tetroxide 12:24 Phenylhydrazine and Dinitrogen tetroxide (hypergolic reaction) 13:59 Osmium tetroxide presentation 15:32 Anhydrous hidrazine and Osmium tetroxide! 16:40 Vanadium oxytrichloride presentation 17:33 Anhydrous hidrazine and Vanadium oxytrichloride 19:46 Anhydrous hidrazine and liquid chlorine 22:04 Anhydrous hidrazine and bromine 23:04 Anhydrous hidrazine and Cobalt trifluoride 24:39 Anhydrous hidrazine and Iodine pentoxide 25:53 A Big thanks to my dear patrons and donors! ❤❤❤ ❤️ 💛 💚 If you enjoy what I do and would like to help me to create unique chemical content please support me on Patreon 🥺 __________ Patreon: https://www.patreon.com/ChemicalForce PayPal: @chemicalforce Revolut: @chemicalforce __________ crypto: BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2Ethereum ❤️ 💛 💚

iqVsKWMmMe0 | 13 Dec 2022
Hey! I'm almost done with the video about anhydrous hydrazine and I hope to publish it on Monday (December 19, 2022). ❤️ 💛 💚 Creating these videos is very expensive and hard. But your support helps me a lot! __________ Patreon: https://www.patreon.com/ChemicalForce PayPal: @chemicalforce Revolut: @chemicalforce __________ crypto: BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2Ethereum ❤️ 💛 💚

m9O0Ha3jFNM | 29 Nov 2022
Hey! While I'm working on a new video about anhydrous hydrazine I decided to post some footage of a classic reaction. Nothing out of the ordinary but better than nothing :D 🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥 REACTION TIMING 0:00 Intro 0:54 Reaction preparation 1:23 Thunderstorm in a test tube 1:34 Manganese heptoxide and ethanol reaction 4:17 Burning phosphorus under water 4:50 Ultra slow-mo underwater explosions 5:34 Chlorosulfonic acid instead of sulfuric acid 6:03 Triple bubble explosion 7:31 BLOOPER :DDD 🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥 ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: @chemicalforce ❤️ 💛 💚 Subscribe bro!

3cvUXW__QZ0 | 17 Nov 2022
I made a real iodine cloud ☁ 🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥 0:00 Iodine trichloride and hydrazine hydrate reaction 0:26 Iodine trichloride and hydrazine hydrate reaction in the sulfur hexafluoride atmospere! 1:23 Making a real iodine cloud! 2:46 Titanium tetrachloride and water reaction 2:57 Making a white cloud from titanium tetrachloride 3:39 The most beautiful rust formation you’ve ever seen 6:24 Lithium and sulfur hexafluoride reaction Li + SF6 🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥 ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: @chemicalforce ❤️ 💛 💚 Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ❤️ 💛 💚

zF81-Wd51Pk | 18 Oct 2022
When heated, alkali metal azides decompose with evolution of nitrogen and an alkali metal VAPORS! 🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥 0:00 Sodium azide decomposition 0:59 Sodium azide decomposition in slow-mo 3:19 Brown-orange sodium vapors 3:43 Potassium azide decomposition 4:46 Potassium azide decomposition in slow-mo 4:33 Bluish-green potassium vapors 7:37 Qualitative reaction to azide ion 8:48 Thanks a lot to my dear patrons ❤️ 🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥 ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: @chemicalforce ❤️ 💛 💚

2MpZleo3p6I | 27 Sep 2022
You all know about such inorganic compound as lead dichloride PbCl2, but there’s also lead tetrachloride PbCl4 that I’m going to talk about in this video! Feel free to leave your comments, I will be glad to read them all! 🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥 0:00 Lead dichloride demonstration 0:14 Hexachloroplumbic Acid synthesis H2PbCl6 1:31 Triethylammonium hexachloroplumbate synthesis 2:41 Lead tetrachloride synthesis 4:09 Lead tetrachloride photolysis 4:33 Lead tetrachloride decomposition when heated 5:12 Lead tetrachloride decomposition on a hot plate 7:12 Lead tetrachloride hydrolysis 7:52 Lead dioxide and liquid hydrogen sulfide reaction 8:44 PbCl4 + H2S liquid 8:55 Liquid hydrogen sulfide trailer 9:11 Thanks a lot to my dear patrons ❤️ 🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥 ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce ❤️ 💛 💚

ZhBIlOeaLgw | 07 Sep 2022
My flask is destroyed by hydrogen and ozone explosion 😰 🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥 REACTION TIMING 0:00 Pop Test Hydrogen 0:06 Platinum powder demonstration 0:27 Hydrogen oxidation on platinum 1:13 Hydrogen oxidation on platinum (thermal imager) 1:23 Hydrogen and oxygen explode in the test tube 2:25 Hydrogen and oxygen explode in the three-neck flask 3:47 Hydrogen and OZONE explode in the three-neck flask 🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥 ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon! 😍 ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce ❤️ 💛 💚 Ko-Fi: https://ko-fi.com/chemicalforce PayPal: @chemicalforce ❤️❤️❤️ Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ❤️❤️❤️ Subscribe bro!

QNEx-__I0n0 | 26 Aug 2022
Not my best video 😁, but it will answer the question "Does ammonia burn in air?" 🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥 REACTION TIMING 0:00 Burning ammonia in air 0:06 Extinguishing a fire with liquid ammonia 0:18 Extinguishing a fire with liquid methane 0:35 RIP mosquito 0:56 Ammonia and oxygen jet flame 1:38 Ammonia and ozonated oxygen jet flame 2:27 Ammonium ozonide forming 2:58 Liquid ammonia and Manganese heptoxide NH3 + Mn2O7 3:40 Liquid ammonia and permanganyl fluoride NH3 + MnO3F 4:24 Catalytic oxidation of ammonia with chromium (III) oxide NH3 + Cr2O3 5:18 Catalytic oxidation of ammonia with vanadium (V) oxide NH3 + V2O5 6:15 Chromyl chloride can ignite ammonia NH3 + CrO2Cl2 7:59 This chemical reaction looks like the spread of mold 🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥 ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon! 😍 ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce ❤️ 💛 💚 Ko-Fi: https://ko-fi.com/chemicalforce PayPal: [email protected] ❤️❤️❤️ Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ❤️❤️❤️ Subscribe bro!

1cQZAfSNA-k | 08 Aug 2022
I Soaked Burning Magnesium in iodine-containing organic liquids! 🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥 REACTION TIMING 0:00 Intro 0:37 Methyl iodide demonstration 1:00 Methyl iodide and liquid oxygen combustion 2:20 Methyl iodide and burning magnesium 4:22 Methylene iodide burning 4:34 Methylene iodide and liquid oxygen combustion 5:04 I Soaked Burning Magnesium in the heaviest organic liquid in the world :D 5:55 Ethyl iodide demonstration 6:34 Allyl iodide demonstration 7:32 Iodoform demonstration 7:52 Iodoform and burning magnesium reaction 8:10 Tetrabromomethane 🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥 ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon! 😍 ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce ❤️ 💛 💚 Ko-Fi: https://ko-fi.com/chemicalforce PayPal: [email protected] ❤️❤️❤️ Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ❤️❤️❤️ Subscribe bro!

I6fOCNT9ufY | 26 Jun 2022
🌩 A mushroom cloud 12:33 🐙 Octopus: 19:50 Hey guys! In this video I will show you unique chemical reactions with cobalt and iron carbonyls that you have never seen before (as always) 😄. Cobalt and Iron carbonyls formed an octopus🐙 and fire cloud as a result of a chemical reaction. Dicobalt octacarbonyl, also known as Co2(CO)8, is a highly reactive compound with a unique set of chemical properties. In this video, we will take a closer look at the reactivity of dicobalt octacarbonyl, and explore some of its important applications in synthetic chemistry. You'll learn about the key features that make dicobalt octacarbonyl such a valuable tool in the laboratory. Whether you're a student of chemistry, or simply someone who is interested in the fascinating world of chemical reactions, this video will give you a deeper understanding of dicobalt octacarbonyl and its place in the field of chemistry. 0:00 Intro 0:55 ASMR Chemistry :D 1:05 Dicobalt Octacarbonyl demonstration and reaction with air 2:40 Cobalt carbony + fire 🔥 3:04 Co2(CO)8 decomposition in air 3:36 Co2(CO)8 decomposition in inert atmosphere 4:39 Сobalt dissolves in hydrochloric acid 7:41 Drying cobalt carbonyl with thionyl chloride 9:55 Cobalt carbonyl and nitric acid 10:24 Cobalt carbonyl and halogens (Br2, I2) 11:23 Cobalt carbonyl and tert-butyl hydroperoxide 12:33 Iron pentacarbonyl and tert-butyl hydroperoxide 15:16 Chromyl chloride and Iron pentacarbonyl 15:55 What the hell is that!? 16:07 Make a solution of cobalt carbonyl in iron carbonyl 18:25 Dark solution + Sodium peroxide 18:49 Dark solution + Potassium superoxide 19:50 I made an octopus 🐙 21:10 Other "octopuses" ❤️ 💛 💚 Creating these videos is very expensive and hard. But your support helps me a lot! Patreon: https://www.patreon.com/ChemicalForce Ko-Fi: https://ko-fi.com/chemicalforce PayPal: @chemicalforce Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2

8hrYlhTYl5U | 31 May 2022
With hydrogen, boron forms a series of dangerous and highly reactive compounds that can explode when reacting with other chemicals. 🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥 0:00 Decaborane, Borazabe and o-Carborane presentation. 0:14 Ammonia borane intro 1:28 Ammonia borane combustion in liquid oxygen 2:17 Ammonia borane as hydrogen storage 2:33 Catalytic decomposition of ammonia borane with platinum 3:36 Borazane and osmium tetroxide 4:23 Explosive reaction between ammonia borane and fuming nitric acid 5:16 ortho-Carborane presentation 5:34 o-Carborane combustion 7:18 o-Carborane and fuming nitric acid 8:04 Decaborane presentation 8:25 Decaborane combustion in liquid oxygen 8:49 Solid-solid reaction! Decaborane and Potassium superoxide 9:56 Detonating reaction between Decaborane and Osmium tetroxide 10:30 The most beautiful chemical reaction in slow-mo 😄 13:16 Extremely dangerous reaction with detonation between Decaborane and HNO3 100% 15:49 Patrons ❤️ 🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥 ❤️ 💛 💚 Creating these videos is very expensive and hard. But your support helps me a lot! Patreon: https://www.patreon.com/ChemicalForce Ko-Fi: https://ko-fi.com/chemicalforce PayPal: @chemicalforce ❤️ 💛 💚

W_j9UKYyoHs | 20 May 2022
A few chemical reactions with chlorosulfonic acid HSO3Cl 🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥 REACTION TIMING: 0:00 Intro 0:42 Opening 0:52 Thermal decomposition 1:55 Hydrolysis 2:37 Chlorosulfonic acid and ice 3:05 Chlorosulfonic acid and egg white 4:56 A spectacular reaction with lithium aluminum hydride LiAlH4 5:25 Chlorosulfonic acid and potassium HSO3Cl + K 6:12 Chlorosulfonic acid and maghesium HSO3Cl + Mg 7:07 Chlorosulfonic acid and tin HSO3Cl + Sn 7:42 Chlorosulfonic acid and selenium 8:16 Chlorosulfonic acid and iodine 8:34 HSO3Cl + KNO3 9:00 Chlorosulfonic acid and table salt 9:27 Chlorosulfonic acid and silver nitrate 10:04 Molten silver nitrate ignites the filter paper 11:02 Patrons ❤️ 🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥 ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: @chemicalforce Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ❤️ 💛 💚

a_hiwU67HpM | 10 May 2022
Suddenly I got the idea to mix potassium permanganate and fluorosulfuric acid to get PERMANGANYL FLUORIDE (MnO3F) and I did it right away! KMnO4 + 2HSO3F → MnO3F + KSO3F + H2SO4 Permanganyl Fluoride: A Brief History of the Molecule MnO3F and of Those Who Cared For It https://d-nb.info/1229250409/34 ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ❤️ 💛 💚

Ss-gkibkTJs | 22 Apr 2022
In this video we'll get Cl2O6 by pouring dilute H2SO4 to the mixture of oxalic acid and KClO3 REACTION TIMING: 🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥 0:00 Dichlorine Hexoxide making 2:08 Freshly made Cl2O6 testing 4:09 Dichlorine Hexoxide + Lithium Aluminum Hydride 4:34 Dichlorine Hexoxide + Acetone 5:03 Dichlorine Hexoxide + Methyl acrylate 5:45 not fresh Cl2O6 testing 7:10 Dichlorine Hexoxide + Decaborane 7:32 Carbon disulfide + Chlorine dioxide (CS2+ClO2) 7:38 Dichlorine Hexoxide + Carbon disulfide (CS2+Cl2O6) 8:33 Dichlorine Hexoxide + Molten sulfur 9:17 Dichlorine Hexoxide + NH3 liquid 9:55 Patrons ❤️ 🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥 ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon! 😍 ❤️ 💛 💚 Creating these videos is very expensive and hard. But your support helps me a lot! Patreon: https://www.patreon.com/ChemicalForce Ko-Fi: https://ko-fi.com/chemicalforce PayPal: @chemicalforce Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ❤️ 💛 💚

Fa3I7KCnY8c | 01 Apr 2022
Cigarette + Liquid Oxygen = Rocket? Cigarette filter soaked in liquid oxygen burns with a jet flame, that stream is definitely capable of a slight payload motion. ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon! 😍 ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 Ethereum ETH: 0x6E21d103dAe2bC577AF9F89CC325cB3B675245EC ❤️ 💛 💚

kulzx3wKm4s | 27 Mar 2022
In this video we’ll be looking at chemical reactions through the lens of thermal camera. 🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥 REACTION TIMING: 0:00 Intro 0:48 Sulfuric acid, oleum and chlorosulfonic acid hydrolysis 1:49 Hydrogen peroxide and potassium permanganate H2O2 + KMnO4 2:38 Volcano 😀 3:10 Dissolving ammonium nitrate in water, with decreasing temperature 3:53 Liquid ammonia + Liquid hydrogen chlorine 5:57 Liquid ammonia + Liquid chlorine 7:08 Bug 🪲 🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥 ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon! 😍 ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 Ethereum ETH: 0x6E21d103dAe2bC577AF9F89CC325cB3B675245EC ❤️ 💛 💚

gqcu3yEkQ7g | 14 Mar 2022
🎂 It’s anniversary time!🎂 💯This is my hundredth video!💯 😻Thank you for staying with me all this time!😻 😲 Wow! I can’t believe I’ve filmed all this stuff! 🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥 REACTION TIMING: 0:00 Сesium demonstration 3:40 I break a cesium ampoule 6:28 Cesium and water reaction 7:18 Cesium and FUMING NITRIC ACID (~100%) 7:55 Caesium and Fluorosulfonic acid (HSO3F) 8:37 Cesium and Bromine 9:22 Caesium and Iodine monochloride 10:55 Caesium and Iodine trichloride 11:48 Dissolution of cesium in liquid ammonia 12:51 Сesium in liquid ammonia and Iodine monochloride 13:27 Caesium and chloroform 🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥🔥 ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon! 😍 ❤️ 💛 💚 Creating these videos is very expensive and hard. But your support helps me a lot! Patreon: https://www.patreon.com/ChemicalForce Ko-Fi: https://ko-fi.com/chemicalforce PayPal: @chemicalforce Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ❤️ 💛 💚

ViMocu_iPOU | 19 Feb 2022
🎬 In this video I will show you a few very cool reactions with acetylene, liquid chlorine and ozone. 💥🔥There will be photochemical reactions, liquid gases, fire and explosions, and you’ll see them all in slow-mo⏳. Just the way you like it :D ____________________________ 0:00 Intro 0:22 Solid acetylene and Potassium permanganate solution C2H2 + KMnO4 0:53 Photochemical reaction with bleach and calcium carbide 💥⏳ 2:43 Photochemical Reaction of Hydrogen and Chlorine H2+Cl2 💥⏳ 4:19 Solid acetylene and Chlorine vapors C2H2 + Cl2 🔥⏳ 5:26 Solid acetylene and Liquid Chlorine 💥⏳ 6:31 Combustion of acetylene in liquid oxygen 🔥 6:50 Explosion of acetylene in liquid oxygen 💥⏳ 7:21 Combustion of phenylacetylene in liquid oxygen C6H5CH + O2 🔥🔥⏳ 8:28 Liquid ozonated oxygen and solid acetylene C2H2 + O2/O3 🔥 9:40 Explosion upon contact of Ozone and Acetylene 💥⏳ 10:35 Unstable ozone explodes in a Petri dish 💥⏳ 12:57 Photochemical explosion of ozone/chlorine/acetylene mixture 💥⏳ ____________________________ ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon! ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2

x1U_ZyMdSIM | 04 Feb 2022
It would be difficult to turn osmium tetroxide into a chemical weapon or "dirty bomb," but it could still do a lot of harm to anyone exposed to it. OsO4 is expensive and highly toxic. The powerful combination of high toxicity and high volatility makes osmium tetroxide very dangerous chemical. ____________________________ 0:00 Osmium tetroxide presentation 0:09 "Dirty bomb" newspaper clippings 0:39 Demonstration of osmium tetroxide in a sealed ampoule 2:27 Demonstration of the volatility of an osmium tetroxide crystal 2:48 Osmium qualitative reactions 4:54 The oxidation of alkenes with osmium tetroxide 5:30 OsO4 + CsOH 6:10 OsO4 + HBr 6:45 EXPLOSIVE REACTIONS WITH OsO4 IN SLOW MO ____________________________ ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon ❤️ 💛 💚 Creating these videos is very expensive and hard. But your support helps me a lot! Patreon: https://www.patreon.com/ChemicalForce Ko-Fi: https://ko-fi.com/chemicalforce PayPal: @chemicalforce Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________

pWB7oGbd8Yo | 24 Dec 2021
MAGIC ACID! "The name originated after a Christmas party in 1966🎉, when a member of the Olah lab placed a paraffin candle into the magic acid, and found that it dissolved quite rapidly." 🎦 55 years later we will repeat this experiment and check this myth ✅ ClO3F: Perchloryl fluoride. ____________________________ ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ 🔔 Subscribe, bro ^__^

xcvw7k_txpM | 15 Dec 2021
The Dangerous Adventures of SpongeBob. Oxygen-Soaked SpongeBob DISAPPEARS instantly! SpongeBob, Liquid Oxygen and Laughing Gas! 😂 ____________________________ ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ 🔔 Subscribe, bro ^__^

qH50C5nXC8U | 07 Dec 2021
You know that red phosphorus is resistant to physical impact as well as in air, but if you try to crack a piece of violet phosphorus (or Hittorf's metallic phosphorus), the fragments may ignite! Actually, you can consider it a low-key red phosphorus, although there will be much more difference in this case, unlike comparing the difference between white and yellow phosphorus ____________________________ ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ 🔔 Subscribe, bro ^__^

njV70etcQYM | 18 Nov 2021
Thanks for watching and joing me for this experiment, hope you enjoyed! ̶S̶o̶r̶r̶y̶ ̶I̶ ̶w̶a̶s̶t̶e̶d̶ ̶y̶o̶u̶r̶ ̶t̶i̶m̶e̶ ̶o̶n̶ ̶t̶h̶i̶s̶ ̶v̶i̶d̶e̶o̶,̶ ̶g̶u̶y̶s̶.̶ ̶A̶n̶d̶ ̶I̶'̶m̶ ̶e̶v̶e̶n̶ ̶m̶o̶r̶e̶ ̶s̶o̶r̶r̶y̶ ̶t̶o̶ ̶h̶a̶v̶e̶ ̶w̶a̶i̶s̶t̶e̶d̶ ̶t̶i̶m̶e̶ ̶o̶f̶ ̶m̶y̶ ̶p̶a̶t̶r̶o̶n̶s̶ ̶t̶h̶a̶t̶ ̶s̶u̶p̶p̶o̶r̶t̶ ̶m̶e̶,̶ ̶s̶o̶ ̶t̶h̶a̶t̶ ̶I̶ ̶c̶a̶n̶ ̶m̶a̶k̶e̶ ̶Q̶U̶A̶L̶I̶T̶Y̶ ̶c̶h̶e̶m̶i̶c̶a̶l̶ ̶c̶o̶n̶t̶e̶n̶t̶,̶ ̶n̶o̶t̶ ̶a̶l̶l̶ ̶o̶f̶ ̶t̶h̶i̶s̶ ̶s̶t̶u̶f̶f̶😂 ____________________________ ICE BALL PRESS: 🌐 https://www.amazon.com/Perfect-Cocktails-creating-crystal-clear-bartenders/dp/B0946X86BV/ 💍 https://www.amazon.com/Cocktails-creating-crystal-clear-DIAMONDS-bartenders/dp/B09417R8XS/ ____________________________ ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ 🔔 Subscribe, bro ^__^

59UC6scLu_c | 14 Nov 2021
Thiophosgene is a suflur analogue of phosgene. It's an orange-red liquid with strong smell! And this reagent is the highlight of today's video! ____________________________ 0:00 Thiophosgene presentation 0:30 Unpacking 1:35 Opening 1:45 Thiophosgene and moist air 2:10 Thiophosgene and mosquito 2:36 Thiophosgene dimerization 4:20 Thiophosgene and aniline water (qualitative reaction) 5:35 Thiophosgene hydrolysis 6:57 Perchloromethyl mercaptan (CSCl2 + Cl2) 7:34 Thiophosgene + Potassium superoxide 7:58 Thiophosgene + pyridine 8:39 Thiophosgene neutralization 9:32 Thiophosgene and liquid ammonia ____________________________ ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ 🔔 Subscribe, bro ^__^

TXELwy11uos | 16 Oct 2021
In this video, I’m going to show you a few reactions with CoF3, MnF3, CeF4 and you’re also going to find out if this is really the way to get elemental fluorine in the laboratory 0:00 presentation of cans 0:58 CoF3 opening 2:05 CoF3 decomposition to F2 2:43 CoF3 + HCl 3:36 CoF3 + NH3*H2O 4:00 CoF3 + Si 6:04 CoF3 + S 6:45 CoF3 + Mg 7:06 CoF3 + Ti 8:16 CoF3 + MnF2 8:53 MnF3 10:12 MnF3 + H2O 10:25 MnF3 decomposition to F2 11:09 F2 + Mg 11:52 MnF3 + Mg 12:16 F2 + Tm 13:05 F2 + H2 13:54 CeF4 15:04 CeF4 + Mg slow-mo 🎥⌚️ Shooting slow-mo video requires a lot of financial costs💵, so if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ 🔔 Subscribe bro ^__^

DNLSUfJYoww | 26 Sep 2021
Hey! In this FUNNY :D video I’m going to carry on with the series of experiments with burning magnesium, changing the liquids I’m putting it into. And all of this is going to be in SLOW-MO. 0:00 Intro 0:28 Hydrogen peroxide 1:47 Liquid ammonia 3:54 HCl 5:00 CH2Cl2 5:32 CHCl3 6:24 CCl4 🎥⌚️ Shooting slow-mo video requires a lot of financial costs💵, so if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ 🔔 Subscribe bro ^__^

gZTtQW5wXJI | 26 Aug 2021
In this video about white phosphorous and various reactions with it you'll also see a lot of slow-mo footages, and we'll find out what will happen if phosphorous ignites on skin. Anyway, make sure you'll watch this video till the end. It will be fascinating! __________ 0:00 Hey guys :D 0:16 Intro :D 0:25 How Phosphorous REALLY glows! 0:35 White phosphorus burning 1:18 White phosphorus burning =-SLOW-MO-= 2:18 Burning phosphorus on the skin 3:41 Burning phosphorus underwater 5:57 Burning magnesium underwater 6:43 White phosphorus purification 10:03 Liquid chlorine and White phosphorus reaction 10:13 Liquid chlorine and White phosphorus reaction =-SLOW-MO-= 11:03 Liquid chlorine and White phosphorus in carbon disulfide solution reaction 11:13 Liquid chlorine and White phosphorus in carbon disulfide solution reaction =-SLOW-MO-= 12:21 Bromine and White phosphorus reaction 12:28 Bromine and White phosphorus reaction =-SLOW-MO-= 13:03 Bromine and White phosphorus in carbon disulfide solution reaction =-SLOW-MO-= 15:34 Iodine and White phosphorus in carbon disulfide solution reaction 16:07 Iodine monochloride and white phosphorus reaction 16:18 Iodine monochloride and white phosphorus reaction =-SLOW-MO-= 16:47 thanks to the sponsor 17:11 Manganese heptoxide and white phosphorus reaction =-SLOW-MO-= 17:33 Potassium chlorate and white phosphorus explosive reaction (KClO3 + P4) 17:49 Potassium chlorate and white phosphorus explosive reaction =-SLOW-MO-= 18:08 Potassium superoxide and white phosphorus explosive reaction =-SLOW-MO-= 18:56 Nitric acid and white phosphorus reaction19:16 Selenous and white phosphorus reaction 20:21 Silver nitrate solution and white phosphorus reaction 21:14 Copper sulfate and white phosphorus reaction __________ 🎥⌚️ Shooting slow-mo video requires a lot of financial costs💵, so if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ 🔔 Subscribe bro ^__^

EGeY_pENSj8 | 30 Jul 2021
A real cool flame that doesn't burn! :D Hey guys! While I'm working on a big video about WHITE phosphorous I decided to post a separate video on HOW TO MAKE A REAL COLD FIRE out of white phosphorous! __________ ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ 🔔 Subscribe, bro ^__^

J2dECQAYXwE | 15 Jul 2021
Hey guys! In this video I'll waste another chemical reagent so you can see interesting chemical reactions :D ==========Reaction timing========== 0:00 Iodine monochloride demonstration 0:46 Iodine monochloride and Titanium (ICl+Ti) 1:25 ICl + Ti in SlowMo 2:57 Iodine monochloride and sodium (ICl + Na) 3:25 ICl + Na in SlowMo 4:05 Iodine monochloride and Potassium (ICl + K) 4:20 ICl + K in SlowMo 5:36 Iodine monochloride and Antimony (ICl + Sb) 5:41 ICl + Sb in SlowMo 6:25 Iodine monochloride and Antimony crystal 6:58 Iodine monochloride hydrolysis (ICl + H2O) 7:22 Iodine monochloride alkaline hydrolysis (ICl + KOH) 7:58 Iodine monochloride and nitric acid (ICl + HNO3) 8:28 Iodine monochloride and hydrazine hydrate (ICl + N2H4*H2O) 8:52 ICl + N2H4*H2O in SlowMo 10:09 Iodine monochloride and Gold __________ ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ 🔔 Subscribe, bro ^__^

weuOX_R8Lwg | 29 Jun 2021
Hey, guys! I am sure that after watching this video you’ll think: “Damn! I’ve never thought that phosphorous glow is that cool!” You don’t believe me, do you? Well, keep watching! __________ ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ 🔔 Subscribe, bro ^__^

qJ14L7thYvE | 08 Jun 2021
If I take bromine vapors to gallium drop, it starts to slip away from them!😱 🎥⌚️ Shooting slow-mo video requires a lot of financial costs💵, I would be glad to have your support on Patreon ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce ____________________________ PayPal: [email protected] Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ 🔔 Subscribe, bro ^__^

zuRl8Yq6SxY | 19 May 2021
The decomposition of hydrogen peroxide ~1000FPS, ~4300FPS. No elephant toothpaste here! *In the community tab, you can suggest your ideas for reactions in slow-mo. 🎥⌚️ Shooting slow-mo video requires a lot of financial costs💵, I would be glad to have your support on Patreon ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce ____________________________ PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ 🔔 Subscribe, bro ^__^

qnVVDYD9S5k | 11 Apr 2021
Hey guys! In today's short video I'm going to use liquid trimethyl borate and NITROUS OXIDE to get green jet fire! ==========REACTION TIMING========== 0:00 liquid oxygen + elemental boron, decaborane (O2(liq.) + B, B10H14) 0:29 Trimethyl Borate burning (B(OMe)3 + O2) 0:47 Trimethyl Borate hydrolysis (B(OMe)3 + H2O) 1:37 Trimethyl Borate + Mn2O7 2:14 Trimethyl Borate + liquid oxygen 2:31 Trimethyl Borate + liquid ozonated oxygen 2:57 Trimethyl Borate + liquid N2O ==========REACTION TIMING========== ____________________________ ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ 🔔 Subscribe, bro ^__^

Q9fH-hUVrjg | 05 Apr 2021
Now I have 3 interhalogen compounds: ICl3, IBr, ICl. This video about Iodine monobromide. Heating iodine with bromine up to the point of the deliquation of the whole mass and its later cooling, results in the formation of an interhalogen compound, known as Iodine monobromide - IBr. ==========REACTION TIMING========== 0:00 Heating iodine with bromine 0:20 Iodine monobromide bottle demonstration 1:17 IBr crystals (macro video) 2:49 IBr + H2SO4 3:18 IBr + KOH 4:06 IBr + N2H4*H2O (hydrazine hydrate) 4:45 IBr + K 5:14 IBr + Cu 5:37 IBr + P 6:13 IBr + Sb 6:58 IBr + Al ==================== ____________________________ ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ 🔔 Subscribe, bro ^__^

hv-t0Kyx9hg | 27 Mar 2021
How to argue with a chemistry teacher and WIN! 0:00 Paradoxical chemical equation#1 2:37 Paradoxical chemical equation#2 ____________________________ ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ 🔔 Subscribe, bro ^__^

My-UAz_KY-c | 21 Mar 2021
I poured liquid oxygen and liquid N2O to decomposing mercury (II) thiocyanate (the pharaoh's serpent)! ==========REACTION TIMING========== 0:00 mercury (II) thiocyanate decomposing 0:27 mercury (II) thiocyanate decomposing slow-motion x5 2:14 mercury (II) thiocyanate decomposing synthesis 2:58 mercury (II) thiocyanate + liquid oxygen! 3:20 mercury (II) thiocyanate + liquid oxygen! slow-motion x5 5:04 mercury (II) thiocyanate decomposing + nitrous oxide! 5:37 mercury (II) thiocyanate decomposing + nitrous oxide! slow-motion x5 8:00 Thanks to my patrons ❤️ ____________________________ ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ 🔔 Subscribe, bro ^__^

CRuUA0ytsjc | 08 Mar 2021
Fe(CO)5 - Iron pentacarbonyl is toxic and like other metal carbonyls, Fe(CO)5 is high flammable! 0:00 That chemical from the previous video 0:41 Unboxing iron pentacarbonyl 3:18 Physical properties of iron pentacarbonyl 4:30 Determination of carbon monoxide with iodine pentoxide (CO + I2O5) 4:50 Iron from Fe(CO)5 5:10 Getting extra pure 99.99% Iron 7:42 Iron pentacarbonyl burning 10:23 Chemical properties of Fe(CO)5 13:36 Photolysis of Fe(CO)5 17:36 Answer a question about another chemical from the community tab 18:22 Tert-butyl hydroperoxide reacts with CrO3 and Mn2O7 19:52 t-BuOOH hypergolic reaction with iron pentacarbonyl 20:40 Thanks to my patrons ❤️ ____________________________ ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ 🔔 Subscribe, bro ^__^

6_W0hKDOpL0 | 07 Feb 2021
Some unverified sources mention chemiluminescence of ammonia and chromyl chloride: ".... It is somewhat remarkable that few people have observed the beautiful chemiluminescence exhibited by the interaction of chlorine or chromylchloride with ammonia, although I suppose the former reaction is demonstrated annually in at least one of the classes in every school where chemistry is taught …" bc In this short video I’m going to show you what happens, when ammonia contacts chromyl chloride ____________________________ ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ 🔔 Subscribe, bro ^__^

hHinp3QxHEQ | 26 Jan 2021
What happens when anhydrous liquid hydrogen chloride contacts anhydrous liquid ammonia! ____________________________ ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ 🔔 Subscribe, bro ^__^

vttsvMKQ1Yw | 19 Jan 2021
In this video, I’ll show you a very interesting frame I managed to capture, while making a video about the explosive decomposition of ozone into oxygen! ____________________________ 0:00 Hey guys! :D 0:19 My ozone generator 1:27 O3 + KI (solution) 1:46 O3 + KI (solid) 2:15 O3 + Mn2+ ions 2:48 Ozonated oxygen demonstration 3:02 Abrupt decomposition of liquid O3 3:12 Liquid ozonated oxygen + KI 3:40 Liquid ozonated oxygen + AgNO3 4:48 Liquid ozone drops 5:37 That's how liquid ozone explodes ____________________________ Ozone decomposition. Interdisciplinary Toxicology, 7(2), 47–59. doi:10.2478/intox-2014-0008 https://doi.org/10.2478/intox-2014-0008 ____________________________ ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ 🔔 Subscribe, bro ^__^

UWBNcMyfiGQ | 17 Dec 2020
This is not a clickbait! This is that very first video about the strongest acid in the world on YouTube! FluoroantImonic acid! HSbF6 0:00 Iintroduction 1:08 Intro :D 1:17 Fluoroantimonic acid can opening 2:51 Fluoroantimonic acid package opening 3:16 Fluoroantimonic acid PFA bottle demonstration 3:55 What is PFA 4:37 HSbF6 laboratory storage 4:49 Opening HSbF6 bottle 5:36 Glove test 7:42 HSbF6 interaction with paper 7:53 HSbF6 interaction with sawdust 9:15 HSbF6 interaction with skin! 10:34 HSbF6 interaction with meat 11:12 HSbF6 interaction with bone 12:11 HSbF6 interaction with water 13:34 HSbF6 interaction with candle 13:47 Pentavalent carbon 15:00 HSbF6 interaction with benzene 15:55 Benzene + i-C5H12 17:02 HSbF6 + Mg 17:37 HSbF6 + Na 18:10 HSbF6 + K 19:06 Unpacking arrived chemicals 20:14 HSbF6 interaction with tert-Butyllithium (superbase) 23:05 HSbF6 + CsOH 24:02 "Reaction between protons and electrons H+ + e-" 24:43 Dissolving sodium in liquid ammonia (Na + NH3(liq.)) 25:05 HSbF6 interaction with sodium in liquid ammonia solution 25:25 HSbF6 + NaH 26:12 Thanks to patrons ============================== https://pubs.acs.org/doi/abs/10.1021/ja00856a034 https://pubs.acs.org/doi/pdfplus/10.1021/ja00810a037 ============================== ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ 🔔 Subscribe, bro ^__^

g3uIFYmKxYU | 05 Nov 2020
LiAlH4 + GeCl4 → GeH4↑ + LiCl + AlCl3 In this video you’ll be able to see germane, even though it’s colorless and you can’t feel its awful smell through your display. We will get germane by the reaction of lithium aluminum hydridie in dry diethyl ether and germanium tetrachloride. ________________________________________ ✔️ If you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ 🔔 Subscribe, bro ^__^

mHhs5OdNCJw | 30 Oct 2020
Hey, guys! The thing is, I just finished making footage about germane (GeH4)! Where the main precursor was germanium tetrachloride, and, before I upload it to the channel, I’d like to show you a couple of reactions with germanium (IV) chloride and germanium (IV) iodide. ________________________________________ ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ 🔔 Subscribe, bro ^__^

JOqTNmHaRo8 | 10 Oct 2020
*Yay! You just saw HSbF6 for the first time on YouTube :DD Today I’ll show a couple of archive videos, that I made a long time ago but haven’t used anywhere yet. ________________________________________ 0:00 HSbF6!!!! for the first time on YouTube!!! 0:15 Thanks to my patrons 0:32 Be + liquid chlorine reaction 2:13 "Breaking Bad" reaction 2:27 Barium demonstration 3:20 Ba + Br2 3:37 Re2O7 demonstration 4:03 Weighing of Re2O7 and Hf powder 4:15 Tungsten plate (foil) demonstration 4:30 Pouring the mixture (Re2O7+Hf) 4:39 2Re2O7 + 7Hf = 4Re + 7HfO2 5:27 Yttrium + liquid oxygen (Y + O2) ________________________________________ ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ 🔔 Subscribe, bro ^__^

ukeq48CXuzI | 01 Oct 2020
Some time ago I came across the article that claimed, that the interreaction of alkaline earth metal oxides with oleum results in chemiluminescence. Well, I decided to put it to the test myself! *To be honest, I noticed a tiny flash only in BaO and MgO reactions, and the latter is the only one I managed to get right on camera :( ___ http://www.chem-page.de/experimente/chemolumineszenz-mit-erdalkalieoxiden.html ___ S. Albrecht, H. Brandl und T. Zimmermann. Anorganische Chemilumineszenz. Traditionelle Experimente in neuem Licht. Chem. unserer Zeit 2008, 42 (6), 394–400. DOI: 10.1002/ciuz.200800460 ____________________________ ✔️ If you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ 🔔 Subscribe, bro ^__^

MWeIDj3s-RQ | 25 Sep 2020
In this video, I’ll show you a couple of interesting chemical reactions with PBr5 - phosphorus pentabromide. On contact with red phosphorous, liquid bromine sets it on fire, forming such reaction products as phosphorus tribromide - PBr3 and Phosphorus pentabromide - PBr5. PBr5 is a yellow powder, that this video will be about. ============================== ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ 🔔 Subscribe, bro ^__^

Sitk5nB6uiY | 14 Sep 2020
Hey guys! I got HSbF6 - The world's strongest acid! 🆘 Please, share your ideas of reactions with fluoroantimonic acid in the comments! I think, you’ll have some great suggestions, that I’ll be able to carry out, like alkylation of benzene with methane, or what will happen if you dip a pH indicator strip in Fluoroantimonic acid... ============================== ✔️ So if you enjoy what I do, and would like to help me to buy chemical reagents and equipment, as some of my viewers do, I will be glad to see you as a member of my Patreon ❤️ 💛 💚 Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ 🔔 Subscribe, bro ^__^

eMJDK_kmrxw | 27 Jul 2020
Get ready to look at this polymerization for a long time :D ________________________________________ 0:00 Dry benzoyl peroxide demonstration 0:23 Dry benzoyl peroxide explosion 0:50 Benzoyl peroxide melting 1:56 Slightle wet benzoyl peroxide explosion 2:15 Сombustion of commercially available benzoyl peroxide 2:53 Handbook of reactive chemical hazards 3:04 Benzoyl peroxide + aniline 3:30 Benzoyl peroxide + phenylhydrazine 4:02 Benzoyl peroxide + triethylamine 4:32 Methyl acrylate demonstration 5:10 Removal of the stabilizer with alkali 6:12 Adding benzoyl peroxide to methyl acrylate 6:37 Heating for initiation 6:56 Free-Radical polymerization! 7:40 Free-Radical polymerization mechanism 8:46 Acceleration of polymerization at a later stage 9:22 Photoinduced radical polymerization! 11:33 Taking out the polymer from the test tube 11:54 Demonstration of polymethyl acrylate 12:52 Destruction of polymethyl acrylate 13:24 Demonstration of low molecular weight polymers of polymethyl acrylate 14:56 Words of gratitude for my patrons :-* ________________________________________ Support this channel! Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ Subscribe, bro ^__^

8UWZBxpPxFw | 08 Jul 2020
Chemical reactions with Molybdenum pentachloride: 0:00 Mo + O2 2:03 Mo + Cl2 3:25 MoCl5 + O2 5:06 MoCl5 heating 6:44 MoCl5 + O2 (for a long time) 7:57 MoCl5 + H2O 8:54 MoCl5 + H2O (Hydrolysis) 9:48 MoCl5 + HCl 12:17 MoCl5 + HNO3 13:01 MoCl5 + SnCl2 14:23 MoCl5 + LiBH4 (Hypergolic reaction!) ____________________________ Support this channel! https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ Subscribe, bro ^__^

bX33R5uk-Kc | 30 Jun 2020
In this short video I’ll show you what happens, when liquid chlorine contacts liquid ammonia: liquid Cl2 + liquid NH3. Is chloramine formed? Let me know in the comments, what you think about this! ____________________________ Support this channel! https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ Subscribe, bro ^__^

n1kZ4yKNbfg | 26 Jun 2020
In the previous video I showed you an inorganic red chemiluminescence with potassium permanganate: https://www.youtube.com/watch?v=gSP_953YMF8 This time, it will be an inorganic yellow chemiluminescence. This chemiluminescence is much brighter than the one from the previous video, and it can even be seen in the light. ____________________________ RIDEAL, E. Chemiluminescence1. Nature 123, 417–419 (1929). https://doi.org/10.1038/123417a0 Anorganische Chemilumineszenz. Traditionelle Experimente in neuem Licht. https://doi.org/10.1002/ciuz.200800460 ____________________________ Support this channel! https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ Subscribe, bro ^__^

gSP_953YMF8 | 22 Jun 2020
In this video I’ll show you an INORGANIC chemiluminescence, which is a very rare phenomenon in inorganic chemistry. Chemiluminescence is generated directly by excited manganese (II) ions (λ ≈ 690 nm) _______________________________ Adcock, J. L., Francis, P. S., Smith, T. A., & Barnett, N. W. (2008). The characteristic red chemiluminescence from reactions with acidic potassium permanganate: further spectroscopic evidence for a manganese(II) emitter. The Analyst, 133(1), 49–51. doi:10.1039/B714147E https://pubs.rsc.org/en/content/articlelanding/2008/AN/B714147E#!divAbstract _______________________________ Support this channel! https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 _______________________________ Subscribe, bro ^__^

y1ZzlAeOx4k | 26 May 2020
In this video I’ll show you five new ways to make fire in a lab without matches, that you probably won’t use anymore. You’ll just know you have them :D ____________________________ Support this channel! https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) ____________________________ Subscribe bro ^_^

vCEfdKv4mXE | 09 May 2020
Sodium amide, commonly called sodamide is the inorganic compound with the formula NaNH2. This solid, which is dangerously reactive toward water, is white, but commercial samples are typically gray due to the presence of small quantities of metallic iron from the manufacturing process. ____________________________ Support this channel! https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ Subscribe bro ^_^

ctGNK_0SjOg | 24 Apr 2020
I became curious about what would happen if liquid ammonia is poured onto red-hot particles of Chromium(III)Oxide? NH3 + Cr2O3 ____________________________ Support this channel! https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) ____________________________ Subscribe bro ^_^

EHLdn2QJOlk | 04 Apr 2020
Hey, guys! This video doesn't pursue an educational goal this time, but these reactions are very bright, rich in colour and quite impressive nonetheless. Enjoy! :D ____________________________ Support my channel! https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) ____________________________ e-mail: [email protected] ____________________________ Subscribe bro ^_^

ao4b_pEiYno | 29 Mar 2020
In this video I mix dry liquid ammonia and almost-pure liquid ozone, that leads to the formation of unstable ammonium ozonide NH4O3! ____________________________ Support my channel! https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) ____________________________ Subscribe bro ^_^

WhcfBLop5gM | 18 Mar 2020
Ozonides filmed with top-notch depth of field! Brought to you by my new professional lighting equipment that I bought thanks to your support! ____________________________ Support my channel! https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Skrill: [email protected] ____________________________ Subscribe bro ^_^

O5LLxVJo8TU | 02 Mar 2020
In this video you'll see another one interesting example of a photochemical reaction: 1,4-Dioxane + Cl2 Here is the first one in case you haven't seen it: https://www.youtube.com/watch?v=P5EJa8hj3Vo Reactions: Dioxane + liquid oxygen Dioxane + liquid ozonated oxygen Dioxane + liquid chlorine ____________________________ Support my channel! https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Skrill: [email protected] ____________________________ Subscribe bro ^_^

r0KkxpHP99c | 22 Feb 2020
In this video I'll crash an ampoule with rubidium in air. May I say that I have a CRUSH on it? :D Ah, never mind, just watch the video... ____________________________ Support my channel! https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Skrill: [email protected] Subscribe bro ^_^

2-CbgjdFZ8g | 01 Feb 2020
Patreon: https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 Supporting me on patreon lets me buy expensive reagents from sigmaaldrich :D This is my 60th video and it will be about interhalogen compound. I know you often ask to show ClF3, but it's too exotic even for this channel (at least for now, sorry). So, today I will show you reactions with iodine trichloride ICl3 ________________ Reaction timing: 1:58 ICl3 = ICl + Cl2 (ICl3 decomposition) 2:48 ICl3 + H2O (ICl3 hydrolysis) 3:20 ICl3 + KOH 3:48 I- + IO3- + H+ 4:30 ICl3 + SnCl2 5:55 ICl3 + P 6:32 ICl3 + LiBH4 7:49 ICl3 + B10H14 9:18 ICl3 + N2H4*H2O ________________ Subscribe, bro! ^_^

BEg0wYqy4hU | 09 Jan 2020
This video about titanium hydride’s reversible decomposing into titanium and hydrogen: TiH2 ⇄ Ti + H2 This is not something "WOW", but it also took me not much time to make this video :D

xnVijYba9k0 | 06 Jan 2020
In this video I’ll show you the reaction of a NaK alloy with Br2! This channel has already reached 10K subscribers! This year we’ll see really cool chemical reactions! ____________________________ Support this channel! https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ Subscribe bro ^_^

ySmGMxKC-kg | 26 Dec 2019
Explosions&Fire2: https://www.youtube.com/channel/UCVovvq34gd0ps5cVYNZrc7A/videos Hey, guys! I collaborated with Explosions&Fire channel, so make sure you watch all the video so you can see very cool chemical reactions! Boron tribromide BBr3 is yellowish liquid smokes in the air very actively, forming fog from hydrogen bromide. _________________ Reaction timing: 0:42 BBr3 + H2O 1:04 BBr3 + H2SO4 2:18 BBr3 + KOH 2:47 BBr3 + NH3*H2O 3:15 Na + O2 3:33 BBr3 + Na 4:08 BBr3 + K 4:38 H3BO3 + C2H5OH 4:55 BBr3 + C2H5OH 5:45 BBr3 + CH3OH 7:40 BBr3 + LiH (https://youtu.be/qDxQIzOMV0w) _________________ Subscribe, bro ^_^

iX5uPvi33Zo | 09 Dec 2019
Ammonium perchlorate (NH4ClO4) is a powerful oxidizer! The primary use of ammonium perchlorate is in making solid fuel propellants ================ Reaction timing: 0:44 Ammonium perchlorate (NH4ClO4) decomposition 1:54 Ammonium perchlorate and Zirconium powder NH4ClO4 + Zr 2:05 Ammonium perchlorate and Hafnium powder NH4ClO4 + Hf 2:47 Ammonium perchlorate, magnesium and polyurethane NH4ClO4 + Mg + [C(O)-NH-Ph-CH2-Ph-NH-C(O)-O-C2H4-O-]n 4:24 Ammonium perchlorate and Decaborane NH4ClO4 + B10H14 4:52 Ammonium perchlorate and Lithium borohydride NH4ClO4 + LiBH4 5:55 Ammonium perchlorate and Lithium aluminum hydride NH4ClO4 + LiAlH4 ================ ____________________________ Support this channel! https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2 ____________________________ Subscribe bro ^_^

jtTLS6Vj3xc | 26 Nov 2019
Bromine and non-metals reactions! Maybe they all will seem too similar to you, but, nevertheless, nobody has ever shown them on YouTube until now. ==================== Reaction timing: 1:20 Br2 + Ge (Bromine and germanium) 3:34 Br2 + Sb (Bromine and antimony) 5:58 Br2 + Si (Bromine and silicon) 8:00 Br2 + B (Bromine and boron) 8:53 BBr3 demonstration 9:17 Br2 + P (Bromine and phosphorus) 9:59 PBr3 and PBr5 demonstration ==================== Subscribe, bro ^__^

E-CglpXyYXQ | 13 Nov 2019
The most toxic selenium compound! Despite the fact that it is the most toxic selenium compound, it is very difficult to get poisoned by it because of its tendency to oxidize with the formation of much less toxic red selenium. So, I will get hydrogen selenide through the reaction of sodium selenide with hydrochloric acid and the hydrolysis of aluminum selenide. ============== Subscribe, bro ^_^

tcLBGDBu2l8 | 11 Oct 2019
What Happens if You Drop Potassium Metal in Decomposing Hydrogen Peroxide? O2+H2=BOOM or NOT? https://www.youtube.com/watch?v=kUhIj9Stp1Q - The Action Lab "What Happens if You Drop Sodium Metal in Elephant Toothpaste?"

4ejCVDLCEMc | 07 Oct 2019
Hey, guys! In this video I will demonstrate the chemical reaction between carbon monoxide and nitrogen dioxide. Both are toxic gases produced by decomposition of toxic chromium hexacarbonyl. ============================= Thanks for watching! Subscribe, bro! ^_^

NBpXb77tZyA | 02 Oct 2019
Hey, guys! This video about chromium hexacarbonyl Cr(CO)6. WARNING!!! Chromium hexacarbonyl is toxic and thought to be carcinogenic! Be careful while working with this chemical and don't forget to subscribe! ____________ Like Music (cdk Mix) by Analog By Nature (c) copyright 2015 Licensed under a Creative Commons Attribution Share-Alike (3.0) license. http://dig.ccmixter.org/files/cdk/48915 Ft: Phasenwandler

Z6_ge_Ee9lQ | 06 Sep 2019
EXTRA-concentrated sulfuric acid and sugar reaction! ============= Hey, guys! I made a video about oleum, it will be next! Since the reaction of sulfuric acid and sugar is very popular, I decided to make the reaction of oleum and sugar, and see how users of Reddit vote for it. Spoiler alert: very bad :| SUBREDDIT: https://www.reddit.com/r/chemicalreactiongifs/ MY PROFILE: https://www.reddit.com/user/yourchemicalforce Original video (H2SO4 + sugar): https://www.youtube.com/watch?v=xK4z_YhtTBM

pjC2VrqTdhw | 29 Aug 2019
https://www.youtube.com/watch?v=IuTvfppaf7c - Don't Drop Sodium Metal in Sulfuric Acid! (The Action Lab channel). Hey, guys! Two weeks ago The Action Lab channel has uploaded a video where James drops a chunk of sodium into sulfuric acid. I decided I would recreate this experiment, but this is ChemicalForce, so that's why I will use much more reactive potassium instead of sodium, and a high percentage of oleum instead of sulfuric acid! Subscribe, bro! ^_^

VPohbSeiImA | 26 Aug 2019
https://www.youtube.com/channel/UCCk-tkYf3ctaDcb4EzWVWvw - Random Experiments Int. - Experiments and syntheses ================================ Hello, guys! In this video: Solid N2O and CS2 reaction. Liquid N2O and elementary boron (N2O + B) SUPEREXOTIC REACTION: Liquid N2O and decaborane - propellant mixture! Enjoy! ;)

N1GVb5jGnmE | 07 Aug 2019
I hope that eventually I will be able to show you the reactions with all nitrogen oxides , and in this video there will be reactions with its lower oxide - nitrous oxide, laughing gas N2O! What other reactions with nitrous oxide would you like to see on this channel? Let me know in the comments!

l6mstvvQEnM | 23 Jul 2019
Hello, guys! In this video there will be just two unique chemical reactions with liquid gases! -The first chemical reaction is the one of liquid carbon monoxide with nitrous oxide (CO + N2O) -The second chemical reaction: Liquid hydrogen sulfide and dinitrogen monoxide (H2S+N2O) ============ Subscribe guys!

zjl0hxsQgxU | 26 Jun 2019
just pouring liquid nitrogen to burning lithium ========== Reaction timing: 0:55 Burning lithium + liquid nitrogen (Li + N2) 1:40 Burning lithium + Dinitrogen monoxide vapors (Li + N2O) 2:53 Burning lithium + liquid oxygen (Li + O2) 3:28 Burning lithium + liquid chlorine (Li + Cl2) ========== Thank's for watching! ;)

P5EJa8hj3Vo | 18 Jun 2019
Hello, guys! In this video you'll see a quite vivid example of a photochemical reaction! Thank's for watching! ===== Don't forget to subscribe ^_^ ===== ____________________________ Support my channel! https://www.patreon.com/ChemicalForce PayPal: [email protected] (Shcherba) Skrill: [email protected] ____________________________

kM8Wf1Xn1VU | 02 Jun 2019
Hey, guys! In this video we will burn some NON-metals in liquid oxygen! ========== Reaction timing: 0:20 Liquid oxygen + Carbon (C + O2) 1:30 Liquid oxygen + Sulfur (S + O2) 4:30 Liquid oxygen + Red phosphorus (P + O2) 5:20 Liquid oxygen + Silicon (Si + O2) 6:50 Liquid oxygen + Boron (B + O2) 8:56 Liquid oxygen + Tellurium (Te + O2) ========== Thank's for watching! Don't forget to subscribe ^_^

qGaBg0s1B8E | 14 May 2019
This video may be not as interesting as the previous ones, but now they are released more often :D Hey, guys! In this video we will burn some metals in liquid oxygen! ========== Reaction timing: 0:57 Liquid oxygen + Calcium metal 1:56 Liquid oxygen + Magnesium metal 2:43 Liquid oxygen + Strontium metal 3:39 Liquid oxygen + Barium metal 4:40 Liquid oxygen + Samarium metal 5:28 Liquid oxygen + Titanium metal ========== Thank's for watching! Don't forget to subscribe ^_^

xqcrN82tNf4 | 07 May 2019
Liquid oxygen and Highly Flammable Organic Liquids is a cool combo! ========== Reaction timing: 0:46 Liquid oxygen + Carbon disulfide 1:20 Liquid oxygen + Nitromethane 2:27 Liquid oxygen + Acetonitrile 3:11 Liquid oxygen + Thiophene 3:40 Liquid oxygen + Hexaethyldisilane 4:28 Liquid oxygen + Methyl iodide 6:04 Liquid oxygen + Phenylacetylene ========== Thank's for watching! Don't forget to subscribe ^_^

tJ5tTfZfjFk | 30 Apr 2019
Hey, guys! This video is about the reactions with liquid oxygen. The one about exotic thermits with Re2O7 is coming soon...mb =) ========== Reaction timing: 0:10 Burning cigarettes in liquid oxygen 0:53 LiH + O2 (Lithium hydride and liquid oxygen) 1:18 LiAlH4 + O2 (Lithium aluminum hydride and liquid oxygen) 1:53 LiBH4 + H2O (Lithium borohydride and water) 2:07 LiBH4 + O2 (Lithium borohydride and liquid oxygen) 2:58 KBH4 + O2 (Potassium borohydride and liquid oxygen) 3:39 NaBH4 + O2 (Sodium borohydride and liquid oxygen) 4:36 B10H14 + O2 (Decaborane and liquid oxygen) ========== Thank's for watching! Don't forget to subscribe ^_^

5dlnnoCNVlY | 02 Apr 2019
Hello, guys! Our guest today is a very rare chemical reagent! ========== Reaction timing: 0:26 Re2O7 demonstration of Re2O7 1:15 melting and sublimation of rhenium heptoxide 1:50 rhenium heptoxide crystallization 3:32 Hydrolysis of boron tribromide (BBr3 + H2O) 3:51 Re2O7 + HBr 5:15 Re2O7 + H2S 5:48 Re2O7 + H2S 6:07 Re2O7 * 2L (L=1,4-dioxane) 6:38 decomposition of Re2O7 * 2L (L=1,4-dioxane) 7:06 ReO3 + O2 7:39 ReO3 + HNO3 7:52 Re2O7 + H2O 8:13 HReO4 + Mg 8:34 HReO4 + N2H4 9:00 Qualitative reaction for rhenium 9:46 Extraction 10:40 Rhenium peroxide formation (but it is not exactly) ========== Thank's for watching! Don't forget to subscribe ;)

3ad5TjGGX94 | 17 Mar 2019
Hello, guys! Sorry for a long absence ^_^ I'm work a lot now to make new videos come out more often, so you have to wait. Sorry once again. ========== Reaction timing: 0:35 I2O5 decomposition (2I2O5 = 2I2 + 5O2) 1:10 HIO3 forming (I2O5 + H2O) 1:40 Mg + HIO3 2:07 HCOOH + H2SO4 (carbon monoxide forming) 2:24 Carbon monoxide burns (CO + O2) 2:40 I2O5 + CO 5:10 I2O5 + HCl 5:44 I2O5 + N2H4⋅H2O 6:18 I2O5 + Al 6:26 I2O5 + S 7:05 I2O5 + C12H22O11 7:25 I2O5 + C 7:35 I2O5 + LiBH4 ========== What chemical reaction did you like the most? Thank's for watching! Don't forget to subscribe ;)

9Mre7T-9IMs | 08 Feb 2019
Hello guys! When I edit another video with chemical reactions I don't use some of the footage for different reasons: camera shake, defocus, my decision as a director, etc. In this video you'll see the compilation of RANDOM 5 CHEMICAL REACTIONS, I once filmed that didn't make to the main videos. PLEASE Don't forget to vote for the reaction you liked the most in the COMMUNITY tab. ================================== NO REACTION TIMING FOR THIS RUBRIC. ================================== THANKS FOR WATCHING!

2AdlfdWD4f0 | 30 Jan 2019
Hey, guys! This video doesn't have that much meaning. Just three chemical reactions with decaborane made for fun. I had nowhere to add them, so I decided to upload them on the channel separately, to make sure they won't get lost. Let me know what you think about it in the comments. If you really like it I'll post random reactions more often. Thank you! ========== Reaction timing: 0:25 KMnO4 + H2SO4 0:35 Mn2O7 + CS2 0:41 Mn2O7 + B10H14 0:55 KMnO4 + H2SO4 1:25 B10H14(CS2 solution) + Mn2O7 1:42 B10H14 + Ba(BrO3)2 2:09 B10H14 + LiBH4 ========== Thank's for watching!

Dl7dcJnz6Mc | 26 Jan 2019
*Sorry for delaying new video and some defocus. Hello guys! In this video you'll see some hypergolic reactions with chromyl cloride (CrO2Cl2). ========== Reaction timing: 1:07 Chromyl chloride + lithium aluminum hydride (CrO2Cl2 + LiAlH4) 1:31 Chromyl chloride + diethyl ether (CrO2Cl2 + Et2O) 2:00 Chromyl chloride + morpholine (CrO2Cl2 + (CH2)2O(CH2)2NH 2:18 Chromyl chloride + styrene (CrO2Cl2 + PhCHCH2) 2:33 Chromyl chloride + phenylacetylene (CrO2Cl2 + PhCCH) 3:18 Chromyl chloride + hydrazine hydrate 100% (CrO2Cl2 + N2H4⋅H2O) 5:00 Chromyl chloride + phosphorus trichloride (CrO2Cl2 + PCl3) ========== Thanks for watching! and subscribing *)

UrUqPIFep1s | 07 Dec 2018
White phosphorus solution and barking dog reaction...You won't see it in this video! ========== Reaction timing: 1:15 CS2 + O2 (carbon disulfide combastion reaction) 1:45 Carbon disulfide air blast 2:01 Xanthogenate reaction 3:03 MoO3 + NH3*H2O 4:27 Dissolution of iodine in carbon disulfide 4:40 Sodium azide decomposition NaN3 5:50 NaN3 + I2 7:41 Dissolution of sulfur in carbon disulfide 7:54 Cu + S 8:47 Cool chemical reaction 9:30 Bonus reaction with chlorine dioxide ========== Thanks for watching! and subscribing :)

aDh8-l7DDbM | 23 Nov 2018
Some chemical reactions with metallic samarium. ========== Reaction timing: 0:35 Sm + H2O 1:19 Sm burning in air 1:44 Sm + O2 2:18 Sm + Cl2 2:54 Sm + Br2 3:10 Sm + I2 3:35 Sm + S 4:03 Sm + KO2 4:20 Sm + BaO2 4:32 Sm + HNO3 5:10 Cu + HNO3 5:14 Sm + NO2 5:51 Sm + N2O3 ========== Youtube unlocked the COMMUNITY tab on this channel!

uPB80LUdzvU | 10 Nov 2018
Hey guys! Nitrosyl perchlorate reactions! Enjoy! ========== Reaction timing: 0:48 HNO3 + (C6H10O5)n (starch) 0:55 NO + NO2 + HClO4 2:18 NOClO4 + H2O 2:43 NOClO4 decomposition 3:33 NOClO4 + CH3COCH3 4:00 NOClO4 + Et2O 4:22 NOClO4 + CO(NH2)2 4:40 NOClO4 + Ti 5:10 NOClO4 + LiBH4 5:52 NOClO4 + Sm ========== You can suggest your ideas for reactions, what kind of chemicals do you want to see, etc. in the comments! ========== Thanks for watching!

ndMDK_UHv-U | 31 Oct 2018
This video contains some reactions from subscribers. Enjoy. ========== Reaction timing: 0:56 KO2 + Zr (Potassium superoxide and Zirconium powder) 1:20 KO2 + Ti (Potassium superoxide and Titanium powder) 1:36 KO2 + Mg (Potassium superoxide and Magnesium powder) 1:49 KO2 + P (Potassium superoxide and red phosphorus) 2:06 KNO3 + P (Potassium nitrate and red phosphorus) 2:33 KO2 + SOCl2 (Potassium superoxide and Thyonil chloride) 3:00 KMnO4 + H2SO4 (Potassium permanganate and sulfuric acid) 3:21 Mn2O7 + CS2 (Manganese heptoxide and carbon disulfide) ========== You can suggest your ideas for reactions, what kind of chemicals do you want to see, etc. in the comments! ========== Thanks for watching!

3u9VzoOxPeU | 20 Oct 2018
This video about preparation diborane from sodium borohydride and Tin (II) chloride. When heated with no solvent, SnCl2 (anhydrous) and NaBH4 produce B2H6 with high yield (up to 94%). ========== Reaction timing: 0:33 NaBH4 + O2 1:05 KBH4 + O2 1:33 NaBH4 + H2SO4 2:08 KBH4 + H2SO4 2:32 NaBH4 + SnCl2(anhydrous) 4:30 B2H6 + O2 5:01 NaBH4 + SnCl4(anhydrous) ========== Thanks for watching!

TNreVYIyWCE | 12 Oct 2018
Hello guys! As I promised, in this video I’m gonna show you chemical reactions with SF6: Sulfur hexafluoride, ‘cause, everyone only does deep voice and aluminium foil boats with it, no chemistry! So I’m gonna do it! ========== Reaction timing: 1:00 Lithium burning (Li + O2 + N2) 1:23 Li + SF6 2:18 Lithium metal + sulfur hexafluoride (jet) 2:52 Lithium metal + sulfur hexafluoride (jet) slowmo 3:34 Li + SF6(excess) 4:03 Mg + SF6 4:25 Ti + SF6 5:00 Ca + SF6 6:10 Sr + O2 (strontium burning) 6:51 Ba + SF6 7:34 Samarium burning (Sm + O2) 7:42 Sm + SF6 8:16 Frozen sulfur hexafluoride demonstration (SF6 molten) 8:48 Li + SF6 frozen 9:42 Magnesium + Sulfur hexafluoride frozen ========== Thanks for watching, guys!

VlpYy5WywE0 | 13 Sep 2018
This video about chemical reactions with superoxide of potassium (KO2). ========== Reaction timing: 0:49 KO2 + H2O (hydrolysis of potassium superoxide) 1:22 KO2 + CO2 (chemical oxygen generator) 2:09 KO2 + nitromethane (CH3NO2) 2:40 KO2 + formic acid (HCOOH) 3:06 KO2 + formamide (HCONH2) 3:43 KO2 + 2,4-dinitrophenylhydrazine 4:25 KO2 + carbon disulfide (CS2) 4:55 KO2 + phosphorus (V) chloride (PCl5) 5:48 KO2 + decaborane (B10H14) ========== More subscribers = more exotic chemical reactions :D thx for watching!

Sc8sNw4QQXY | 17 Aug 2018
Hi, guys. Some your chemical suggestions in this video. I saw very cool suggestions (like Mn2O7+N2H4) but I want to use it in individual video about some chemical substance. ========== Reaction timing: 0:20 Beryllium burning Be + O2 1:03 KMnO4 + H2SO4 1:28: Mn2O7 + P 2:37 Zr + P 3:22 Zr + KClO4 4:52 Ti + B 5:50 Mn2O7 + LiAlH4 ========== You can suggest your ideas for reactions, what kind of chemicals do you want to see, etc. under this video. Thx for watching!

2WAZ2bWiMT8 | 11 Jul 2018
Hello guys! More beautiful chemical reactions with titanium! ========== Reaction timing: 0:29 Burning titanium wire 0:40 Burning titanium powder 1:01 Burning Titanium symbol "Ti" 1:30 Titanium and hydrofluoric acid Ti + HF 2:17 Titanium and Iodine reaction: Ti + I2 2:59 Titanium and bromine reaction: Ti + Br2 4:25 Titanium and ammonium perchlorate: Ti + NH4ClO4 4:55 Titanium and hydrozine perchlorate: Ti + N2H5ClO4 5:20 Hydozine perchlorate and potassium periodate: N2H5ClO4 + KIO4 6:09 Titanium and sulfur reaction: Ti + S 7:16 Titanium and phosphorus reaction: Ti + P 8:10 Titanium and dinitrogen trioxide: Ti + N2O3 ========== Thanks for watching! Don't forget to leave your suggestions in the comment section below.

3o3pICtWkv0 | 29 Jun 2018
Hello guys! Thionyl chloride is our guest today :D ========== Reaction timing: 1:02 Thionyl chloride is hydrolyzed (SOCl2 + H2O) 1:44 Thionyl chloride decomposes 2:25 Sulfur dichloride and Disulfur dichloride are hydrolyzed (SCl2 + H2O and S2Cl2 + H2O) 3:00 Cobalt (II) chloride hexahydrate dehydration by thionyl chloride CoCl2*6H2O + SOCl2 4:50 Cobalt (II) chloride monohydrate hydration 5:37 Thionyl chloride and potassium metal SOCl2 + K 6:08 Thionyl chloride and lithium metal SOCl2 + Li 7:21 Thionyl chloride and barium metal SOCl2 + Ba 7:36 Thionyl chloride and samarium metal SOCl2 + Sm 7:55 Thionyl chloride and titanium metal SOCl2 + Ti ========== Thanks for watching! Subscribe ;)

wrLM2fvseos | 21 Jun 2018
Hello guys! In this video we verify Heisenberg's reaction with red phosphorus that he used to escape from the gangsters. ========== Reaction timing: 0:43 Red phosphorus burning on the air 1:05 Red phosphorus burning and liquid oxygen 2:13 Mg + P 2:56 Magnesium phosphide burning 3:14 Magnesium phosphide formation 3:22 Magnesium phosphide and water reaction (Mg3P2 + H2O) 3:57 Phosphine formation 4:11 Let's cook :) ========== Thanks for watching and subscribing ;)

0YFM0jFm3Jc | 28 May 2018
In this video the reactions you suggested. I try to make more interactive :) ========== Reaction timing: 0:27 LiAlH4 + -(C2F4)n- 1:29 LiAlH4 + H2SO4 2:13 LiAlH4 + (NH4)2Cr2O7 3:19 P + KClO4 4:40 B10H14 + Mn2O7 ========== Some reactions I post in reddit before Youtube Follow me on reddit: https://www.reddit.com/user/yourchemicalforce/ ========== You can suggest your ideas for reactions, what kind of chemicals do you want to see, etc. in the comments ========== Thx!

GXf1vys5IRA | 01 Apr 2018
Some reactions with methane, nitromethane and acetylene. ========== Reaction timing: 0:26 solid acetylene evaporation 0:46 solid acetylene burning C2H2 + O2 air 1:05 liquid methane burning CH4 + O2 air 1:17 liquid methane + liquiid oxygen CH4 + O2 1:50 nitromethane + liquid oxygen CH3NO2 + O2 2:15 nitromethane + decaborane CH3NO2 + B10H14 3:25 solid acetylene + liquid oxygen C2H2 + O2 3:55 solid acetylene + liquid chlorine C2H2 + Cl2 4:42 silver acetylide decomposition Ag2C2 + bright light ========== Follow me on reddit https://www.reddit.com/user/yourchemi... 500px: https://500px.com/chemicalforce ========== Thanks for watching! You can suggest your ideas for reactions, what kind of chemicals do you want to see, etc. in discussion tab or under this video.

KB_jnuTs91I | 17 Mar 2018
This video has a pack of reactions with Decaborane(14) ========== Reaction timing: 1:38 B10H14 burning 2:03 B10H14 burning (macro) 2:35 B10H14 + O2 (gas) 3:10 B10H14 + O2 (liquid) 4:07 B10H14 + KClO3 (mechanic initiation) 4:33 B10H14 + KClO3 (H2SO4 initiation) 4:57 B10H14 + KClO3 (melt) 5:21 B10H14 + Na2O2 (mechanic initiation) 5:49 B10H14 + Na2O2 (H2O initiation) 6:18 B10H14 + Ba(NO3)2 6:52 B10H14 + HNO3 ========== Follow me on reddit https://www.reddit.com/user/yourchemicalforce/ 500px: https://500px.com/chemicalforce ========== Thanks for watching! You can suggest your ideas for reactions, what kind of chemicals do you want to see, etc. in discussion tab or under this video.

bR_LAr_mOCw | 11 Feb 2018
This video about Titanium tetraсhloride (TiCl4) and reactions with it. ========== Reaction timing: 1:35 TiCl4 on the air 2:29 TiCl4 solid 2:57 Ti + Cl2(liq.) 3:59 Ti + Cl2(liq.) slowmo 5:05 TiCl4 add to H2O 5:37 H2O add to TiCl4 6:22 NH3·H2O + TiCl4 (solid) 6:57 NH3·H2O + TiCl4 8:15 TiCl4 + NaBH4 9:06 TiCl4 + NaBH4 slowmo ========== Thanks for watching!

7ZwqumCdyY4 | 21 Jan 2018
Decomposition of sodium azide (NaN3) and lead azide (Pb(N3)2). ========== Reaction timing: 0:45 NaN3 decomposition 1:23 NaN3 decomposition and sodium metallic formation 2:26 Pb(N3)2 decomposition by flame 3:04 Pb(N3)2 decompsition by friction ========== thanks for watching

6tXGwv6T9wI | 14 Jan 2018
Compilation of sodium peroxide cool reactions. Na2O2 or sodium peroxide is a yellow powder that ignites many organic matters by contact. ====================== Sodium peroxide reactions timing: 0:20 Acetic acide (Na2O2+CH3COOH) 0:35 Nitromethane (Na2O2+CH3NO2) 1:24 Ammonium thiocyanate (Na2O2+NH4SCN) 1:51 Lithium aluminum hydride (Na2O2+LiAlH4) 2:22 Zirconium (Na2O2+Zr) 2:36 Titanium (Na2O2+Ti) 3:05 Magnesium (Na2O2+Mg) 3:41 Aluminum (Na2O2+Al) 4:41 Phosphorus (Na2O2+P) 5:18 Sulfur (Na2O2+S) ---------- Bonus 6:02 Na2O2 + intrigue :) 6:41 Na2O2 + intrigue :) Enjoy!

IqlyWd5Q6Ac | 09 Jan 2018
Unique reactions lithium aluminum hydride with different substances. ==================== Reactions timing: 0:23 burning LiAlH4 0:30 burning LiAlH4 (macro) 1:25 decomposition LiAlH4 under a laser ray 1:35 decomposition LiAlH4 due to temperature 2:06 LiAlH4 and water (H2O) 2:33 LiAlH4 and nitric acid (HNO3) 2:50 LiAlH4 and sodium nitrate (NaNO3) 3:16 LiAlH4 and potassium nitrate (KNO3) 3:43 LiAlH4 and rubidium nitrate (RbNO3) 4:20 LiAlH4 and cesium nitrate (CsNO3) 4:52 LiAlH4 and lithium nitrate (LiNO3*2H20) 5:07 LiAlH4 and barium nitrate (Ba(NO3)2) 5:34 LiAlH4 and Iodine (I2)










